Abstract
Introduction
The antipsychotic drug olanzapine is effective against chemotherapy-induced nausea and targets multiple receptors known to be involved in the emetic reflex arch. The drug has a mean half-life of 30 h, which allows for a single daily administration and is therefore of interest in patients with advanced cancer suffering from nausea.
Objectives
To investigate the antiemetic effect and tolerability of olanzapine in patients with advanced cancer not receiving chemotherapy or irradiation.
Methods
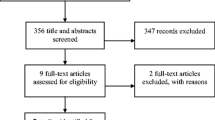
Patients with advanced cancer (no curable treatment options) with at least “moderate” nausea and/or one emetic episode within the last 24 h were included if they had not received chemotherapy or irradiation (last 2 weeks) and had no reversible causes of nausea/vomiting. Patients were administered 10 mg olanzapine daily for 5 days (the first day subcutaneously and the following 4 days orally). Nausea, vomiting, and adverse effects were assessed daily for 7 days. The primary efficacy parameter was nausea after 24 h.
Results
Forty patients from four centers were included and all evaluable after 24 h. Thirty-six patients experienced some degree of improvement. The mean two-item N/V score (0–100) at baseline was 66 and improved to 21 and 24 after 24 h and 7 days, respectively. During the course of the study, the dose of olanzapine was reduced in three patients due to adverse events. Five patients were withdrawn from the study primarily due to progression of malignant disease or per patient’s request.
Conclusions
Olanzapine appears effective and tolerable as an antiemetic in patients with advanced cancer. Future research should examine a lower dose (5 or 2.5 mg), preferably in a randomized controlled trial.



Similar content being viewed by others
References
Glare P, Miller J, Nikolova T, Tickoo R (2011) Treating nausea and vomiting in palliative care: a review. Clin Interv Aging 6:243–259. https://doi.org/10.2147/cia.s13109
Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT (1996) Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14(2):87–96. https://doi.org/10.1016/0893-133x(94)00129-n
Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, Brandt D, Dolan DE, Ellis G, Kim EJ, Kirkegaard S, Kloth DD, Lagman R, Lim D, Loprinzi C, Ma CX, Maurer V, Michaud LB, Nabell LM, Noonan K, Roeland E, Rugo HS, Schwartzberg LS, Scullion B, Timoney J, Todaro B, Urba SG, Shead DA, Hughes M (2017) NCCN guidelines insights: antiemesis, version 2.2017. J Natl Compr Cancer Netw 15(7):883–893. https://doi.org/10.6004/jnccn.2017.0117
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH (2017) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35(28):3240–3261. https://doi.org/10.1200/jco.2017.74.4789
Herrstedt J, Roila F, Warr D, Celio L, Navari RM, Hesketh PJ, Chan A, Aapro MS (2017) 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high emetic risk chemotherapy. Support Care Cancer 25(1):277–288. https://doi.org/10.1007/s00520-016-3313-0
Walsh D, Davis M, Ripamonti C, Bruera E, Davies A, Molassiotis A (2017) 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer 25(1):333–340. https://doi.org/10.1007/s00520-016-3371-3
Passik SD, Navari RM, Jung SH, Nagy C, Vinson J, Kirsh KL, Loehrer P (2004) A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study. Cancer Investig 22(3):383–388
Jackson WC, Tavernier L (2003) Olanzapine for intractable nausea in palliative care patients. J Palliat Med 6(2):251–255. https://doi.org/10.1089/109662103764978506
Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R (2003) Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manag 25(6):578–582
Shinjo T, Okada M (2006) Olanzapine use in cancer patients for refractory vomiting. Gan To Kagaku Ryoho 33(3):349–352
Kitada T, Narimatsu T, Yamaguchi S (2009) Olanzapine as an antiemetic in intractable nausea and anorexia in patients with advanced hepatocellular carcinoma: three case series. [Japanese]. Acta Hepatologica Japonica 50(3):153–158
Navari RM, Brenner MC (2010) Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 18(8):951–956. https://doi.org/10.1007/s00520-009-0739-7
Kaneishi K, Kawabata M, Morita T (2012) Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manag 44(4):604–607. https://doi.org/10.1016/j.jpainsymman.2011.10.023
Murakami N, Tanabe K, Yamatani K, Kitazawa H, Fujikawa Y, Amemiya Y, Shimada M, Kadoya S (2012) Use of orally disintegrating olanzapine tablet for patients with cancerous peritonitis and postoperative gastric cancer receiving home palliative care. Gan To Kagaku Ryoho 39(4):649–652
Atkinson SR (2014) Olanzapine for intractable nausea and vomiting in palliative care patients not receiving chemotherapy. J Palliat Med 17(5):503–504. https://doi.org/10.1089/jpm.2014.0030
Suzuki M, Komuro K, Ohara K (2014) Olanzapine and betamethasone are effective for the treatment of nausea and vomiting due to metastatic brain tumors of rectal cancer. Case Rep Gastroenterol 8(1):13–17. https://doi.org/10.1159/000358044
Kaneishi K, Nishimura K, Sakurai N, Imai K, Matsuo N, Takahashi N, Okamoto K, Suga A, Sano H, Maeda I, Nishina H, Yamaguchi T, Morita T, Iwase S (2016) Use of olanzapine for the relief of nausea and vomiting in patients with advanced cancer: a multicenter survey in Japan. Support Care Cancer 24(6):2393–2395. https://doi.org/10.1007/s00520-016-3101-x
MacKintosh D (2016) Olanzapine in the management of difficult to control nausea and vomiting in a palliative care population: a case series. J Palliat Med 19(1):87–90. https://doi.org/10.1089/jpm.2015.0224
Dev R (2018) Intractable nausea and anorexia with weight loss in a patient with advanced breast cancer. J Cachexia Sarcopenia Muscle 9(1):203
Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Duggan L, Kissling W, Leucht S (2010) Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev (3):Cd006654. https://doi.org/10.1002/14651858.CD006654.pub2
Boettger S, Jenewein J, Breitbart W (2015) Haloperidol, risperidone, olanzapine and aripiprazole in the management of delirium: a comparison of efficacy, safety, and side effects. Palliat Support Care 13(4):1079–1085. https://doi.org/10.1017/s1478951514001059
Sutherland A, Naessens K, Plugge E, Ware L, Head K, Burton MJ, Wee B (2018) Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults. Cochrane Database Syst Rev 9:Cd012555. https://doi.org/10.1002/14651858.CD012555.pub2
Fonte C, Fatigoni S, Roila F (2015) A review of olanzapine as an antiemetic in chemotherapy-induced nausea and vomiting and in palliative care patients. Crit Rev Oncol Hematol 95(2):214–221. https://doi.org/10.1016/j.critrevonc.2015.02.010
Markowitz JS, DeVane CL, Malcolm RJ, Gefroh HA, Wang JS, Zhu HJ, Donovan JL (2006) Pharmacokinetics of olanzapine after single-dose oral administration of standard tablet versus normal and sublingual administration of an orally disintegrating tablet in normal volunteers. J Clin Pharmacol 46(2):164–171. https://doi.org/10.1177/0091270005283839
Elsayem A, Bush SH, Munsell MF, Curry E 3rd, Calderon BB, Paraskevopoulos T, Fadul N, Bruera E (2010) Subcutaneous olanzapine for hyperactive or mixed delirium in patients with advanced cancer: a preliminary study. J Pain Symptom Manag 40(5):774–782. https://doi.org/10.1016/j.jpainsymman.2010.02.017
Groenvold M, Petersen MA, Aaronson NK, Arraras JI, Blazeby JM, Bottomley A, Fayers PM, De Graeff A, Hammerlid E, Kaasa S, Sprangers MAG, Bjorner JB (2006) The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer 42(1):55–64
Petersen MA, Aaronson NK, Arraras JI, Chie WC, Conroy T, Costantini A, Dirven L, Fayers P, Gamper EM, Giesinger JM, Habets EJJ, Hammerlid E, Helbostad J, Hjermstad MJ, Holzner B, Johnson C, Kemmler G, King MT, Kaasa S, Loge JH, Reijneveld JC, Singer S, Taphoorn MJB, Thamsborg LH, Tomaszewski KA, Velikova G, Verdonck-de Leeuw IM, Young T, Groenvold M (2018) The EORTC CAT Core-the computer adaptive version of the EORTC QLQ-C30 questionnaire. Eur J Cancer 100:8–16. https://doi.org/10.1016/j.ejca.2018.04.016
Passik SD, Lundberg J, Kirsh KL, Theobald D, Donaghy K, Holtsclaw E, Cooper M, Dugan W (2002) A pilot exploration of the antiemetic activity of olanzapine for the relief of nausea in patients with advanced cancer and pain. J Pain Symptom Manag 23(6):526–532
EMA (2006) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000115/WC500055207.pdf. Accessed July 2018
Bun S, Yonemori K, Akagi T, Noguchi E, Shimoi T, Shimomura A, Yunokawa M, Shimizu C, Fujiwara Y, Makino Y, Hayashi Y, Tamura K (2018) Feasibility of olanzapine, multi acting receptor targeted antipsychotic agent, for the prevention of emesis caused by continuous cisplatin- or ifosfamide-based chemotherapy. Investig New Drugs 36(1):151–155. https://doi.org/10.1007/s10637-017-0487-3
Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, Nakao M, Sakai H, Nakayama T, Minato K, Arai T, Suzuki K, Shimada Y, Nagashima K, Terakado H, Yamamoto N (2018) A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 23(2):382–388. https://doi.org/10.1007/s10147-017-1200-4
Hocking CM, Kichenadasse G (2014) Olanzapine for chemotherapy-induced nausea and vomiting: a systematic review. Support Care Cancer 22(4):1143–1151. https://doi.org/10.1007/s00520-014-2138-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Permissions from the Danish Medicines Agency and the Local Ethics Committee were obtained before initiation of the study. No study procedures were initiated before a written informed consent had been signed by the patient.
Conflict of interest
This study received funding from the Danish Cancer Society, the IMK Foundation, and Odense University Hospital. The funding sources had no influence on design, conduction, analyses of results, or manuscript writing. The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Harder, S., Groenvold, M., Isaksen, J. et al. Antiemetic use of olanzapine in patients with advanced cancer: results from an open-label multicenter study. Support Care Cancer 27, 2849–2856 (2019). https://doi.org/10.1007/s00520-018-4593-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4593-3