Abstract
Purpose
The objective of the present prospective study was to compare the safety and efficacy of a 12-h method to a 6-h method in chronic cancer pain management.
Materials and methods
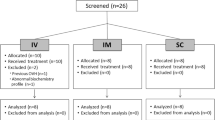
Randomized, prospective clinical trial was conducted between December 2007 and June 2009, enrolling 90 patients with chronic cancer pain. Patients with chronic cancer pain were randomly assigned to the conversion from continuous intravenous infusion to transdermal fentanyl using two-step taper of the continuous intravenous infusion in 12 h (12-h method) or the conversion in 6 h (6-h method). The parameters assessed in the present study included pain intensity (on a scale of 0 to 10) and bolus use frequency, and the adverse effects were assessed with National Cancer Institute Common Terminology Criteria for Adverse Events version 3.
Results
Pain intensity and the number of boluses during conversion remained stable in both arms. The incidence of adverse events was 25.6% in the 12-h method group and 2.3% in the 6-h method group (95% confidence interval, 0.01–0.55; p = 0.002). Adverse events occurred in four patients at 6–12 h, five patients at 12–18 h, two patients at 18–24 h, and one patient at 24–48 h after application.
Conclusions
Excellent safety profile and sustained efficacy are shown for the 6-h conversion method.

Similar content being viewed by others
References
Hanks GW, Justins D (1992) Cancer pain: management. Lancet 339:1031–1036
Mercadante S (1999) Opioid rotation for cancer pain: rationale and clinical aspects. Cancer 86:1856–1866
Radbruch L, Sabatowski R, Loick G, Kulbe C, Kasper M, Grond S, Lehmann KA (2000) Constipation and the use of laxatives: a comparison between transdermal fentanyl and oral morphine. Palliat Med 14:111–119
Morita T, Takigawa C, Onishi H et al (2005) Opioid rotation from morphine to fentanyl in delirious cancer patients: an open-label trial. J Pain Symptom Manage 30:96–103
Duthie DJ, Rowbotham DJ, Wyld R, Henderson PD, Nimmo WS (1988) Plasma fentanyl concentrations during transdermal delivery of fentanyl to surgical patients. Br J Anaesth 60:614–618
Sandler AN, Baxter AD, Katz J, Samson B, Friedlander M, Norman P, Koren G, Roger S, Hull K, Klein J (1994) A double-blind, placebo-controlled trial of transdermal fentanyl after abdominal hysterectomy. Analgesic, respiratory, and pharmacokinetic effects. Anesthesiology 81:1169–1180
Broome IJ, Wright BM, Bower S, Reilly CS (1995) Postoperative analgesia with transdermal fentanyl following lower abdominal surgery. Anaesthesia 50:300–303
Kornick CA, Santiago-Palma J, Khojainova N, Primavera LH, Payne R, Manfredi PL (2001) A safe and effective method for converting cancer patients from intravenous to transdermal fentanyl. Cancer 92:3056–3061
Zech DF, Grond SU, Lynch J, Dauer HG, Stollenwerk B, Lehmann KA (1992) Transdermal fentanyl and initial dose-finding with patient-controlled analgesia in cancer pain. A pilot study with 20 terminally ill cancer patients. Pain 50:293–301
Grond S, Zech D, Lehmann KA, Radbruch L, Breitenbach H, Hertel D (1997) Transdermal fentanyl in the long-term treatment of cancer pain: a prospective study of 50 patients with advanced cancer of the gastrointestinal tract or the head and neck region. Pain 69:191–198
Miser AW, Narang PK, Dothage JA, Young RC, Sindelar W, Miser JS (1989) Transdermal fentanyl for pain control in patients with cancer. Pain 37:15–21
Freynhagen R, von Giesen HJ, Busche P, Sabatowski R, Konrad C, Grond S (2005) Switching from reservoir to matrix systems for the transdermal delivery of fentanyl: a prospective, multicenter pilot study in outpatients with chronic pain. J Pain Symptom Manage 30:289–297
Marier JF, Lor M, Morin J, Roux L, Di Marco M, Morelli G, Saedder EA (2007) Comparative bioequivalence study between a novel matrix transdermal delivery system of fentanyl and a commercially available reservoir formulation. Br J Clin Pharmacol 63:121–124
Paice JA, Cohen FL (1997) Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs 20:88–93
De Conno F, Caraceni A, Gamba A, Mariani L, Abbattista A, Brunelli C, La Mura A, Ventafridda V (1994) Pain measurement in cancer patients: a comparison of six methods. Pain 57:161–166
Frölich MA, Giannotti A, Modell JH (2001) Opioid overdose in a patient using a fentanyl patch during treatment with a warming blanket. Anesth Analog 93:647–648
Shomaker TS, Zhang J, Ashburn MA (2000) Assessing the impact of heat on the systemic delivery of fentanyl through the transdermal fentanyl delivery system. Pain Med 1:225–230
Acknowledgments
We are indebted to Atsushi Komemushi and Takeshi Kodaira for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
None of the authors have identified a conflict of interest.
There are no financial disclosures and no funding considerations from any authors.
Rights and permissions
About this article
Cite this article
Nomura, M., Kamata, M., Kojima, H. et al. Six- versus 12-h conversion method from intravenous to transdermal fentanyl in chronic cancer pain: a randomized study. Support Care Cancer 19, 691–695 (2011). https://doi.org/10.1007/s00520-010-0890-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-010-0890-1