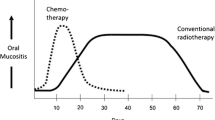
Abstract
Background
With the increased use of so-called targeted anti-cancer therapies, there has been a change in toxicities that patients are experiencing. As most targeted therapies are given in conjunction with more traditional chemotherapeutic agents, toxicities of these combination therapies are also evolving. Whilst we increasingly understand the mechanisms underlying the toxicities of chemotherapy and radiotherapy, the addition of targeted treatments requires a new understanding of both toxicity and interacting mechanisms.
Aims
The aims of this review (which formed the basis of an invited plenary lecture at the 2006 Annual conference of the Multinational Association of Supportive Care in Cancer) were to define targeted anti-cancer therapy, to describe its known impact on the mucosa, either alone, or in combination with chemotherapy with or without radiotherapy, and finally to provide an outline for future directions in research into mucosal injury from targeted anti-cancer therapies.
Methodology
Two separate literature reviews were conducted. The combined reviews produced 700 papers of which approximately 70 were included in the review.
Results
As with mucosal injury (or mucositis) in general, the studies are hampered by a lack of mucosal injury as primary endpoint, and the variable definitions and levels of reporting. The depth to which mucosal injury was studied was also inadequate. However, it is clear that the key to understanding toxicity is to understand the mechanism of action of the drug, from which it should be possible to predict the toxicities that will occur.
Conclusions
With the increasing use of targeted anti-cancer therapies, mucosal injury, particularly in its manifestations of diarrhoea, and mouth ulcers, is becoming even more prominent. More publications of basic and clinical research in this area is required.

Similar content being viewed by others
References
Abigerges D, Chabot GG, Armand JP, Herait P, Gouyette A, Gandia D (1995) Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J Clin Oncol 13:210–221
Adjei AA, Erlichman C, Davis JN, Cutler DL, Sloan JA, Marks RS, Hanson LJ, Svingen PA, Atherton P, Bishop WR, Kirschmeier P, Kaufmann SH (2000) A Phase I trial of the farnesyl transferase inhibitor SCH66336: evidence for biological and clinical activity. Cancer Res 60:1871–1877
Ahmad T, Eisen T (2004) Kinase inhibition with BAY 43-9006 in renal cell carcinoma. Clin Cancer Res 10:6388S–6392S
Alsina M, Fonseca R, Wilson EF, Belle AN, Gerbino E, Price-Troska T, Overton RM, Ahmann G, Bruzek LM, Adjei AA, Kaufmann SH, Wright JJ, Sullivan D, Djulbegovic B, Cantor AB, Greipp PR, Dalton WS, Sebti SM (2004) Farnesyltransferase inhibitor tipifarnib is well tolerated, induces stabilization of disease, and inhibits farnesylation and oncogenic/tumor survival pathways in patients with advanced multiple myeloma. Blood 103:3271–3277
Alvarado Y, Tsimberidou A, Kantarjian H, Cortes J, Garcia-Manero G, Faderl S, Thomas D, Estey E, Giles FJ (2003) Pilot study of Mylotarg, idarubicin and cytarabine combination regimen in patients with primary resistant or relapsed acute myeloid leukemia. Cancer Chemother Pharmacol 51:87–90
Baselga J (2000) Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol 27:20–26
Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK (2004) Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 126:796–808
Borghaei H, Schilder RJ (2004) Safety and efficacy of radioimmunotherapy with yttrium 90 ibritumob tiuxetan (Zevalin). Semin Nucl Med 34:4–9
Borjesson PK, Postema EJ, Roos JC, Colnot DR, Marres HA, van Schie MH, Stehle G, de Bree R, Snow GB, Oyen WJ, van Dongen GA (2003) Phase I therapy study with (186) Re-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with head and neck squamous cell carcinoma. Clin Cancer Res 9:3961S–3972S
Bowen JM, Gibson RJ, Cummins AG, Keefe DMK (2006) Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer 14:713–731
Cappuzzo F, Bartolini S, Ceresoli GL, Tamberi S, Spreafico A, Lombardo L, Gregorc V, Toschi L, Calandri C, Villa E, Crino L (2004) Efficacy and tolerability of gefitinib in pretreated elderly patients with advanced non-small-cell lung cancer (NSCLC). Br J Cancer 90:82–86
Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crino L, Villa E (2004) Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 15:1042–1047
Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, Ostapenko V, Illger HJ, Behringer D, Bardy-Bouxin N, Boni J, Kong S, Cincotta M, Moore L (2005) Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 23:5314–5322
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17:2639–2648
Crane CH, Ellis LM, Abbruzzese JL, Amos C, Xiong HQ, Ho L, Evans DB, Tamm EP, Ng C, Pisters PW, Charnsangavej C, Delclos ME, O’Reilly M, Lee JE, Wolff RA (2006) Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol 24:1145–1151
Curran MP, Croom KF, Goa KL (2004) Spotlight on imatinib mesylate in chronic myeloid leukemia. BioDrugs 18:207–210
Daw NC, Furman WL, Stewart CF, Iacono LC, Krailo M, Bernstein ML, Dancey JE, Speights RA, Blaney SM, Croop JM, Reaman GH, Adamson PC, Children’s Oncology Group (2005) Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol 23:6172–6180
Gibson RJ, Bowen JM, Keefe DM (2005) Palifermin reduces diarrhea and increases survival following irinotecan treatment in tumour-bearing DA rats. Int J Cancer 116:464–470
Gibson RJ, Keefe DM (2006) Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer 14:890–900
Huang FS, Kemp CJ, Williams JL, Erwin CR, Warner BW (2002) Role of epidermal growth factor and its receptor in chemotherapy-induced intestinal injury. Am J Physiol Gastrointest Liver Physiol 282:G432–G442
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Juhl CO, Vinter-Jensen L, Jensen LS, Dajani EZ (1995) Preventive effects of recombinant human epidermal growth factor on the oesophageal epithelium in pigs subjected to sclerotherapy. Eur J Gastroenterol Hepatol 7:823–828
Keefe DM, Gibson RJ, Hauer-Jensen M (2004) Gastrointestinal mucositis. Semin Oncol Nurs 20:38–47
Keefe DM (1998) The effect of cytotoxic chemotherapy on the mucosa of the small intestine. M.D. Thesis, Department of Medicine, University of Adelaide, Adelaide
Kim ES, Kies MS, Fossella FV, Glisson BS, Zaknoen S, Statkevich P, Munden RF, Summey C, Pisters KM, Papadimitrakopoulou V, Tighiouart M, Rogatko A, Khuri FR (2005) Phase II study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in patients with taxane-refractory/resistant nonsmall cell lung carcinoma. Cancer 104:561–569
Larson RA, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL, Mineur P, Bennett JM, Berger MS, Eten CB, Munteanu M, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR, Mylotarg Study Group (2002) Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia 16:1627–1636
Lee DH, Han JY, Lee HG, Lee JJ, Lee EK, Kim HY, Kim HK, Hong EK, Lee JS (2005) Gefitinib as a first-line therapy of advanced or metastatic adenocarcinoma of the lung in never-smokers. Clin Cancer Res 11:3032–3037
Lowndes S, Darby A, Mead G, Lister A (2002) Stevens–Johnson syndrome after treatment with rituximab. Ann Oncol 13:1948–1950
Makower D, Sparano JA, Wadler S, Fehn K, Landau L, Wissel P, Versola M, Mani S (2003) A pilot study of edrecolomab (Panorex, 17-1A antibody) and capecitabine in patients with advanced or metastatic adenocarcinoma. Cancer Invest 21:177–184
Matsuura M, Okazaki K, Nishio A, Nakase H, Tamaki H, Uchida K, Nishi T, Asada M, Kawasaki K, Fukui T, Yoshizawa H, Ohashi S, Inoue S, Kawanami C, Hiai H, Tabata Y, Chiba T (2005) Therapeutic effects of rectal administration of basic fibroblast growth factor on experimental murine colitis. Gastroenterology 128:975–986
Mohamed MK, Ramalingam S, Lin Y, Gooding W, Belani CP (2005) Skin rash and good performance status predict improved survival with gefitinib in patients with advanced non-small cell lung cancer. Ann Oncol 16:780–785
Mokbel K, Hassanally D (2001) From HER2 to herceptin. Curr Med Res Opin 17:51–59
Morelli D, Menard S, Colnaghi MI, Balsari A (1996) Oral administration of anti-doxorubicin monoclonal antibody prevents chemotherapy-induced gastrointestinal toxicity in mice. Cancer Res 56:2082–2085
Nakagawa K, Tamura T, Negoro S, Kudoh S, Yamamoto N, Takeda K, Swaisland H, Nakatani I, Hirose M, Dong RP, Fukuoka M (2003) Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (‘Iressa’, ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol 14:922–930
Playford RJ, Ghosh S, Mahmood A (2004) Growth factors and trefoil peptides in gastrointestinal health and disease. Curr Opin Pharmacol 4:567–571
Punt CJ, Nagy A, Douillard JY, Figer A, Skovsgaard T, Monson J, Barone C, Fountzilas G, Riess H, Moylan E, Jones J, Dethling J, Colman J, Coward L, MacGregor S (2002) Edrecolomab alone or in combination with flurouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet 360:671–677
Robert F, Blumenschein G, Herbst RS, Fossella FV, Tseng J, Saleh MN, Needle M (2005) Phase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy-naive advanced non-small-cell lung cancer. J Clin Oncol 23:9089–9096
Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, Saleh MN, Carey D, LoBuglio AF, Wheeler RH, Cooper MR, Waksal HW (2001) Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol 19:3234–3243
Roberts JD, Shibata S, Spicer DV, McLeod HL, Tombes MB, Kyle B, Carroll M, Sheedy B, Collier MA, Pithavala YK, Paradiso LJ, Clendeninn NJ (2000) Phase I study of AG2034, a targeted GARFT inhibitor, administered once every 3 weeks. Cancer Chemother Pharmacol 45:423–427
Rowinsky EK, Schwartz GH, Gollob JA, Thompson JA, Vogelzang NJ, Figlin R, Bukowski R, Haas N, Lockbaum P, Li YP, Arends R, Foon KA, Schwab G, Dutcher J (2004) Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol 22:3003–3015
Saleh MN, Khazaeli MB, Grizzle WE, Wheeler RH, Lawson S, Liu T, Russel C, Meredith R, Schlom J, LoBuglio AF (1993) A phase I clinical trial of murine monoclonal antibody D612 in patients with metastatic gastrointestinal cancer. Cancer Res 53:4555–4562
Sievers EL (2003) Antibody-targeted chemotherapy of acute myeloid leukemia using gemtuzumab ozogamicin (Mylotarg). Blood Cells Mol Dis 31:7–10
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, Karanes C, Theobald M, Bennett JM, Sherman ML, Berger MS, Eten CB, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR, Mylotarg Study Group (2001) Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 19:3244–3254
Sievers EL, Linenberger M (2001) Mylotarg: antibody-targeted chemotherapy comes of age. Curr Opin Oncol 13:522–527
Sonis ST, Elting LS, Keefe DM, Peterson DE, Schubert MM, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein E, Mucositis Study Section of the Multinational Association for Supportive Care in Cancer, International Society for Oral Oncology (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025
Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC et al (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269:230–234
Tortora GJ, Grabowski SR (eds) (1993) Principles of anatomy and physiology. 7th edn. Harper Collins, New York
Uppenkamp M, Engert A, Diehl V, Bunjes D, Huhn D, Brittinger G (2002) Monoclonal antibody therapy with CAMPATH-1H in patients with relapsed high- and low-grade non-Hodgkin’s lymphomas: a multicenter phase I/II study. Ann Hematol 81:26–32
Veronese ML, Sun W, Giantonio B, Berlin J, Shults J, Davis L, Haller DG, O’Dwyer PJ (2005) A phase II trial of gefitinib with 5-fluorouracil, leucovorin, and irinotecan in patients with colorectal cancer. Br J Cancer 92:1846–1849
Weiner LM (2006) Fully human therapeutic monoclonal antibodies. J Immunother 29:1–9
Yang X, Roskos L, Davis CG, Schwab G (2005) From XenoMouse® technology to Panitumumab (ABX-EGF). In: LaRochelle WJ, Shimkets RA (eds) Cancer drug discovery and development: the oncogenomics handbook, Humana Press, Totowa, pp 647–657
Yeoh AS, Bowen JM, Gibson RJ, Keefe DM (2005) Nuclear factor kappaB (NFkappaB) and cyclooxygenase-2 (Cox-2) expression in the irradiated colorectum is associated with subsequent histopathological changes. Int J Radiat Oncol Biol Phys 63:1295–1303
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keefe, D.M.K., Gibson, R.J. Mucosal injury from targeted anti-cancer therapy. Support Care Cancer 15, 483–490 (2007). https://doi.org/10.1007/s00520-006-0181-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-006-0181-z