Abstract
Goals of work
To conduct an economic analysis comparing tamoxifen and anastrozole (Arimidex) in the adjuvant treatment of hormone receptor-positive (HR+), post-menopausal early breast cancer patients.
Materials and methods
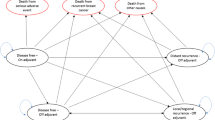
An economic model examined typical patients (64 years of age, HR+, 64% node negative) from the Arimidex, tamoxifen alone, or in combination (ATAC) trial over a lifetime horizon. Rates of events were derived from ATAC trial results. Post-trial event rates were drawn from the literature for tamoxifen; event rates for anastrozole were modified by the relative risks observed in the ATAC trial. Resource utilization was drawn from Statistics Canada’s Population Health Model for breast cancer, supplemented by an expert panel. A public health care system perspective, 2004 Canadian prices and a 5% discount rate were employed.
Results
Anastrozole-taking patients incurred additional hormonal treatment costs compared to tamoxifen-taking patients (incremental lifetime cost, CDN$6,974 per patient), partially offset by reduced downstream recurrences of breast cancer (CDN$1,143 lifetime savings per patient) for a net incremental cost of CDN$5,796 per patient on anastrozole. The anastrozole-treated patients were projected to experience a 5.6% absolute risk reduction of first breast cancer recurrence and a 2.8% absolute risk reduction in breast cancer death. This corresponded to CDN$30,000 per life year gained and CDN$28,000 per quality-adjusted life year gained (95% confidence interval, $17,428 to $54,605). The results were affected by the duration and extent of anastrozole benefit under sensitivity analysis but remained cost-effective.
Conclusion
Compared to tamoxifen, anastrozole therapy is effective and cost-effective as initial adjuvant therapy in post-menopausal, HR+ early breast cancer patients.


Similar content being viewed by others
References
Armstrong K, Chen TM, Albert D, Randall TC, Schwartz JS (2001) Cost-effectiveness of raloxifene and hormone replacement therapy in postmenopausal women: impact of breast cancer risk. Obstet Gynecol 98:996–1003
ATAC Trialists’ Group (2005) Results of the ATAC (Arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60–62
ATAC Trialists’ Group (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer. Results of the ATAC (Arimidex, tamoxifen alone or in combination) trial efficacy and safety update analyses. Cancer 98:1802–1810
ATAC Trialists’ Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Benedict A, Brown RE, Sorensen S (2004) US, UK cost-utility study of anastrozole versus tamoxifen as adjuvant therapy in postmenopausal women with early breast cancer. Medtap International (January)
Boccardo F, Rubagotti A, Amoroso D et al (1992) Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, oestrogen-receptor positive breast cancer patients. An update at 7 years of the 1st GROCTA (Breast Cancer Adjuvant Chemo-hormone Therapy Cooperative Group) trial. Eur J Cancer 28:673–680
Bonneterre J, Buzdar A, Nabholtz JMA et al (2001) Anastrozole is superior to tamoxifen as firstline therapy in hormone receptor positive advanced breast carcinoma: results of two randomized trials designed for combined analysis. Cancer 92:2247–2258
British Columbia Cancer Agency Cancer Management Guidelines. Endometrium. http://www.bccancer.bc.ca/HPI/CancerManagementGuidelines/Gynecology/Endometrium/default.htm
Buzdar AU, Jonat W, Howell A et al (1998) Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma. Results of a survival update based on a combined analysis of data from two mature phase III trials. Cancer 83:1142–1152
Canadian Coordinating Office for Health Technology Assessment (1997) Guidelines for the economic evaluation of pharmaceuticals, 2nd edn. CCOHTA, Ottawa, Canada
Canadian Pharmaceutical Society (2004) Compendium of pharmaceuticals and specialties. Product monograph, Arimidex. Ottawa
Cancer Care Ontario Drug Formulary. http://www.cancercare.on.ca/formulary/regimensitelist1.html#adjuvant
Chia SKL, Speers C, Kang A et al (2003) The impact of new chemotherapeutic and hormonal agents on the survival of women with metastatic breast cancer in a population based cohort. Abstract 22. American Society of Clinical Oncology annual meeting, Chicago (June)
Coombes RC, Hall E, Gibson LJ et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Crowe JP, Gordon NH, Antunez AR et al (1991) Local–regional breast cancer recurrence following mastectomy. Arch Surg 126:429–432
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351:1451–1467
Early Breast Cancer Trialists’ Collaborative Group (1992) Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomised trials involving 31 000 recurrences and 24 000 deaths among 75 000 women. Lancet 339:1–15
Early Breast Cancer Trialists’ Collaborative Group (1988) Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomised trials among 28 896 women. N Engl J Med 319:1681–1692
Fisher B, Costantino J, Redmond C et al (1989) A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumours. N Engl J Med 320:479–484
Fisher B, Dignam J, Wolmark N et al (1997) Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst 89:1673–1682
Fisher B, Dignam J, Bryant J et al (1996) Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 88:1529–1542
Fisher B, Redmond C, Legault-Poisson S et al (1990) Postoperative chemotherapy and tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: results from the National Surgical Adjuvant Breast and Bowel Project B-16. J Clin Oncol 8:1005–1018
Fortin A, Larochelle M, Laverdiere J et al (1999) Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and post-operative radiotherapy. J Clin Oncol 17:101–109
Gail MH, Costantino JP, Bryant J et al (1999) Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst 91:1829–1846
Gold MR, Siegel JE, Russell LB et al (1996) Cost-effectiveness in health and medicine. Oxford Univ. Press, Oxford
Goss PE, Ingle JN, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 19:349–358
Hillner B (2004) Benefit and projected cost-effectiveness of anastrozole versus tamoxifen initial adjuvant therapy for patients with early-stage estrogen receptor-positive breast cancer. Cancer 101:1311–1322
Howell A et al (2003) Effect of anastrozole on bone mineral density: 2-year results of the ‘arimidex’ (anastrozole), tamoxifen, alone or in combination trial. San Antonio breast cancer symposium
Janjan NA, McNeese MD, Buzdar AV et al (1986) Management of locoregional breast cancer. Cancer 58:1552–1556
Lamy A, Yusuf S, Pogue J, Gafni A, HOPE Investigators (2003) Cost implications of the use of ramipril in high-risk patients based on the Heart Outcomes Prevention (Evaluation) HOPE study. Circulation 107:960–965
Laupacis A, Feeny D, Detsky AS et al (1992) How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 146(4):473–481
Leighl NB, Shepherd FA, Kwong R, Burkes RL, Feld R, Goodwin PJ (2002) Economic analysis of the TAX 317 trial: docetaxel versus best supportive care as second-line therapy of advanced non-small cell lung cancer. J Clin Oncol 20:1344–1352
Locker GY (2004) Cost-utility analysis of anastrozole versus tamoxifen as adjuvant therapy in postmenopausal women with early breast cancer from a US healthcare perspective. The ATAC Trialists’ Group (abstract no. 2085). Breast Cancer Res Treat 99(Suppl 1):105
Mansel RE (2005) Cost-utility analysis of anastrozole vs tamoxifen in postmenopausal women with early breast cancer from a UK national health service perspective: the 5-year completed treatment analysis of ATAC (‘Arimidex’, tamoxifen alone or in combination trial (abstract no. 653). J Clin Oncol 23(Suppl 16 pt. 1):41S
Mouridsen H, Gershanovich M, Sun Y et al (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19:2596–2606
O’Brien BJ, Anderson DR, Goeree R (1994) Cost-effectiveness of enoxaparin versus warfarin prophylaxis against deep-vein thrombosis after total hip replacement. CMAJ 150(7):1083–1090
Ontario Case Costing Initiative (2005) Data analyses: average cost per CMG, 2002/2003. http://www.occp.com. Accessed March 10
Ontario Ministry of Health and Long-Term Care (2004) Ontario drug benefit formulary—comparative drug index, version 38. http://www.health.gov.on.ca/english/providers/program/drugs/odbf
Ontario Ministry of Health and Long-Term Care (1999) Schedule of benefits for laboratory services. http://www.health.gov.on.ca/english/providers/program/ohip/sob. April
Ontario Ministry of Health and Long-Term Care (2004) Schedule of benefits—physician services. http://www.health.gov.on.ca/english/providers/program/ohip/sob. Updated November
Paridaens R, Dirix L, Lohrisch C et al (2003) Mature results of a randomized phase II multicentre study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol 14:1391–1398
Pritchard KI, Paterson AHG, Fine S et al (1997) Randomized trial of cyclophosphamide, methotrexate, and fluorouracil chemotherapy added to tamoxifen as adjuvant therapy in postmenopausal women with node-positive estrogen and/or progesterone receptor-positive breast cancer: a report of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 15:2302–2311
Rivkin SE, Green S, Metch B et al (1994) Adjuvant CMFVP versus tamoxifen versus concurrent CMFVP and tamoxifen for postmenopausal, node-positive, and estrogen-receptor positive breast cancer patients: a Southwest Oncology Group study. J Clin Oncol 12:2078–2085
Saphner T, Tormey DC, Gray R (1996) Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 14:2738–2746
Statistics Canada (2002) Complete life table, Canada, 1995–1997, females. http://www.statcan.ca. Accessed October 10
Statistics Canada (2005) Consumer price index. http://www.statcan.ca. Accessed March 10
Wiktorowicz ME, Goeree R, Papaioannou A, Adachi D, Papadimitropoulos E (2001) Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int 12:271–278
Will BP, Berthelot J, Le Petit C et al (2000) Estimates of the lifetime costs of breast cancer treatment in Canada. Eur J Cancer 36:724–735
Will BP, Le Petit C, Berthelot J-M et al (1999) Diagnostic and therapeutic approaches for nonmetastatic breast cancer in Canada, and their associated costs. Br J Cancer 79:1428–1436
Winer EP, Hudis C, Burstein HJ et al (2005) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2005. J Clin Oncol 23:619–629
Acknowledgements
The authors wish to thank Dr. Jean-Marie Berthelot (Statistics Canada) and Dr. Ivo Olivotto (British Columbia Cancer Agency) for their generous sharing of data. This research was partially supported by an unrestricted grant from AstraZeneca Canada
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Summary of major clinical assumptions
-
It was assumed that hormonal therapy was administered for five years, unless patients discontinued therapy.
-
It was assumed that all recurrences and deaths occurred at the mid-point of a year.
-
The rate of first recurrence for tamoxifen, years six to ten, was assumed to be the rate reported by the EBCTCG, for trials with five year intended duration, weighted by nodal status.
-
The rate of first recurrence for anastrozole, years six to ten, was assumed to be the tamoxifen first recurrence rate, modified by the hazard ratio.
-
The rate of first recurrence for tamoxifen, years eleven to fifteen, was assumed to be the mean tamoxifen rate for years six to ten.
-
The rate of first recurrence for anastrozole, years eleven to fifteen, was assumed to be the mean tamoxifen rate for years six to ten.
-
The rate of first recurrence for tamoxifen or anastrozole, years fifteen to thirty-five, was assumed to be that of the general population.
-
The distribution by type of first recurrences, years six to fifteen for tamoxifen, was assumed to follow the ATAC distribution for tamoxifen, years one to five (LRR:DR:CO=25%:60%:15%).
-
The distribution by type of first recurrences, years six to ten for anastrozole, was assumed to follow the ATAC distribution for anastrozole, years one to five (LRR:DR:CO=25%:66%:9%).
-
The distribution by type of first recurrences, years eleven to fifteen for anastrozole, was assumed to follow the ATAC distribution for tamoxifen, years one to five (LRR:DR:CO=25%:60%:15%).
-
The occurrence of DR subsequent to LRR, years six to fifteen, was assumed to be 58%, three years after the LRR.
-
The occurrence of DR subsequent to CO, years six to fifteen, was assumed to be 58%, three years after the CO.
-
Mortality due to breast cancer, years six to fifteen, was assumed to occur only in women with DR, and the mortality rates were those of the BC database rates for Stage IV breast cancer.
-
Mortality due to breast cancer, years sixteen to thirty-five, was assumed to be accounted for by deaths from other causes.
-
Mortality due to adverse events, years one to thirty-five, was assumed to be accounted for by deaths from other causes.
-
It was assumed that adverse events due to hormonal therapy terminated with the discontinuation of hormonal therapy at five years.
Rights and permissions
About this article
Cite this article
Rocchi, A., Verma, S. Anastrozole is cost-effective vs tamoxifen as initial adjuvant therapy in early breast cancer: Canadian perspectives on the ATAC completed-treatment analysis. Support Care Cancer 14, 917–927 (2006). https://doi.org/10.1007/s00520-006-0035-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-006-0035-8