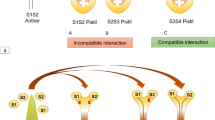
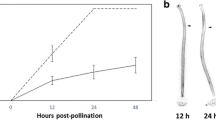
Abstract
The tomato clade within the genus Solanum has numerous advantages for mechanistic studies of reproductive isolation. Its thirteen closely related species, along with four closely allied Solanum species, provide a defined group with diverse mating systems that display complex interspecific reproductive barriers. Several kinds of pre- and postzygotic barriers have already been identified within this clade. Well-developed genetic maps, introgression lines, interspecific bridging lines, and the newly available draft genome sequence of the domesticated tomato (Solanum lycopersicum) are valuable tools for the genetic analysis of interspecific reproductive barriers. The excellent chromosome morphology of these diploid species allows detailed cytological analysis of interspecific hybrids. Transgenic methodologies, well developed in the Solanaceae, allow the functional testing of candidate reproductive barrier genes as well as live imaging of pollen rejection events through the use of fluorescently tagged proteins. Proteomic and transcriptomics approaches are also providing new insights into the molecular nature of interspecific barriers. Recent progress toward understanding reproductive isolation mechanisms using these molecular and genetic tools is assessed in this review.










Similar content being viewed by others
Abbreviations
- SC:
-
Self-compatible
- SI:
-
Self-incompatible
- UI:
-
Unilateral incongruity
- cv:
-
Cultivar
References
Aguilar R, Bernardello G, Galetto L (2002) Pollen–pistil relationships and pollen size-number trade-off in species of the tribe Lycieae (Solanaceae). J Plant Res 115:335–340
Albini SM (1988) Synaptonemal complex spreading in Allium cepa and Allium fistulosum. II. Pachytene observations: the SC karyotype and the correspondence of late recombination nodules and chiasmata. Genome 30:399–410
Albini SM, Jones GH (1984) Synaptonemal complex-associated centromeres and recombination nodules in plant meiocytes prepared by an improved surface-spreading technique. Exp Cell Res 155:589–592
Albini SM, Jones GH, Wallace BMN (1984) A method for preparing two-dimensional surface-spreads of synaptonemal complexes from plant meiocytes for light and electron microscopy. Exp Cell Res 152:280–285
Anderson LK, Covey PA, Larsen LR, Bedinger PA, Stack SM (2010) Structural differences in chromosomes distinguish species in the tomato clade. J Cytogen Genome Res (in press)
Barton DW (1950) Pachytene morphology of the tomato chromosome complement. Am J Bot 37:639–643
Beecher B, McClure BA (2001) Expressing self-incompatibility RNases (S-RNases) in transgenic plants. In: Schein CH (ed) Nuclease methods and protocols. Humana Press, Totowa, pp 65–85
Beecher B, Murfett J, McClure BA (1998) RNaseI from Escherichia coli cannot substitute for S-RNase in rejection of Nicotiana plumbaginifolia pollen. Plant Mol Biol 36:553–563
Bernacchi D, Tanksley SD (1997) An interspecific backcross of Lycopersicon esculentum × L. hirsutum: linkage analysis and a QTL study of sexual compatibility factors and floral traits. Genetics 147:861–877
Borg M, Brownfield L, Twell D (2009) Male gametophyte development: a molecular perspective. J Exp Bot 60:1465–1478
Brown SW (1949) The structure and meiotic behavior of the differentiated chromosomes of tomato. Genetics 34:437–461
Canady MA, Meglic V, Chetelat RT (2005) A library of Solanum lycopersicoides introgression lines in cultivated tomato. Genome 48:685–697
Chandler JM, Jan C-C, Beard BH (1986) Chromosomal differentiation among annual Helianthus species. Syst Botany 11:354–371
Chang S-B, Anderson LK, Sherman JD, Royer SM, Stack SM (2007) Predicting and testing physical locations of genetically mapped loci on tomato pachytene chromosome 1. Genetics 176:2131–2138
Chen K-Y, Cong B, Wing R, Vrebalov J, Tanksley SD (2007) Changes in regulation of a transcription factor lead to autogamy in cultivated tomatoes. Science 318:643–645
Chetelat RT, De Verna JW (1991) Expression of unilateral incompatibility in pollen of Lycopersicon pennellii is determined by major loci on chromosomes 1, 6, and 10. Theor Appl Genet 82:704–712
Chetelat RT, Pertuze RA, Faundez L, Graham EB, Jones CM (2009) Distribution, ecology and reproductive biology of wild tomatoes and related nightshades from the Atacama Desert region of northern Chile. Euphytica 167:77–93
Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59:547–572
Covey PA, KondoK, Welch L, Frank E, Kumar A, Knaap Evd, Nunez R, Lopez-Casado G, Rose JKC, McClure BA, Bedinger PA (2010) Multiple features that distinguish unilateral incongruity and self-incompatibility in the tomato clade. Plant J. doi:10.1111/j.1365-313X.2010.04340.x
Coyne JA, Aulard S, Berry A (1991) Lack of underdominance in a naturally occurring pericentric inversion in Drosophila melanogaster and its implications for chromosome evolution. Genetics 129:791–802
Cruden R (2009) Pollen grain size, stigma depth, and style length: the relationships revisited. Plant Syst Evol 278:223–238
Cruden RW, Lyon DL (1985) Correlations among stigma depth, style length, and pollen grain size: do they reflect function or phylogeny? Botanical Gazette 146:143–149
Dai S, Wang T, Yan X, Chen S (2007) Proteomics of pollen development and germination. J Proteome Res 6:4556–4563
Darwin C (1897) The different forms of flowers on plants of the same species. D. Appleton and Company, London
Dawe RK (2005) Centromere renewal and replacement in the plant kingdom. Proc Natl Acad Sci USA 192:11573–11574
Delphino F (1867) Sull’opera, la distribuzione dei sessi nelle piante e la legge che osta alla perennita della fecundazione consanguinea. Atti Soc Itl Sci Natil 10:272–303
Dobzhansky T (1936) Studies on hybrid sterility II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21:113–135
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141:1147–1162
Garcia CC (2007) Pollen starch reserves in tomato relatives: ecophysiological implications. Grana 46:13–19
Georgiady M, Lord S, Elizabeth M (2002) Evolution of the inbred flower form in the currant tomato, Lycopersicon pimpinellifolium. Int J Plant Sci 163:531–541
Gillies CB (1981) Electron microscopy of spread maize pachytene synaptonemal complexes. Chromosoma 83:575–591
Gottschalk W (1954) Die Chromosomenstruktur der Solanaceen unter Berücksichtigung (relation). Chromosoma 6:539–626
Graham EB, Shannon SM, Persrsen JP, Chetelat RT (2003) A self-compatible population of Lycopersicon peruvianum collected from N. Chile. Tomato Genet Coop 53:22–24
Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang R, Roschitzki B, Basler K, Ahrens CH, Grossniklaus U (2009) Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res 19:1786–1800
Haerizadeh F, Wong C, Bhalla P, Gresshoff P, Singh M (2009) Genomic expression profiling of mature soybean (Glycine max) pollen. BMC Plant Biol 9:25
Hancock CN, Kent L, McClure BA (2005) The stylar 120 kDa glycoprotein is required for S-specific pollen rejection in Nicotiana. Plant J 43:716–723
Hardon JJ (1967) Unilateral incompatibility between Solanum pennellii and Lycopersicon esculentum. Genetics 57:795–808
Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, Hasegawa Y, Ueguchi-Tanaka M, Matsuoka M (2008) Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol 49:1429–1450
Hobo T, Suwabe K, Aya K, Suzuki G, Yano K, Ishimizu T, Fujita M, Kikuchi S, Hamada K, Miyano M, Fujioka T, Kaneko F, Kazama, T, Mizuta Y, Takahashi H, Shiono K, Nakazono M, Tsutsumi N, Nagamura Y, Kurata N, Watanabe M, Matsuoka M (2008) Various spatiotemporal expression profiles of anther-expressed genes in rice. Plant Cell Physiol 49:1417–1428
Hogenboom NG (1972) Breaking breeding barriers in Lycopersicon. 5. The inheritance of the unilateral incongruity between L. peruvianum (L.) Mill. and L. esculentum Mill. and the genetics of its breakdown. Euphytica 21:405–414
Holle M, Rick CM, Hunt DG (1978–1979) Catalog of collections of green-fruited Lycopersicon species and Solanum pennellii found in watersheds of Peru. Tomato Genet Cooper 28–29, 49–78, 63–91
Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S (2005) Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics 5:4864–4884
Honys D, Twell D (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5:R85
Kadej AJ, Wilms HJ, Willemse MTM (1985) Stigma and stigmatoid tissue of Lycopersicon esculentum Miller
Kerim T, Imin N, Weinman JJ, Rolfe BG (2003) Proteome analysis of male gametophyte development in rice anthers. Proteomics 3:738–751
Kondo K, Yamamoto M, Itahashi R, Sato T, Egashira H, Hattori T, Kowyama Y (2002) Insights into the evolution of self-compatibility in Lycopersicon from a study of stylar factors. Plant J 30:143–153
Lai Z, Nakazato T, Salmaso M, Burke JM, Tang S, Knapp SJ, Rieseberg LH (2005) Extensive chromosomal repatterning and the evolution of sterility barriers in hybrid sunflower species. Genetics 171:291–303
Lee H-S, Huang S, Kao T-h (1994) S proteins control rejection of incompatible pollen in Petunia inflata. Nature 367:560–563
Lee C, Page L, McClure B, Holtsford T (2008) Post-pollination hybridization barriers in Nicotiana section Alatae. Sex Plant Reprod 21:183–195
Lee CB, Kim S, McClure B (2009) A pollen protein, NaPCCP, that binds pistil arabinogalactan proteins also binds phosphatidylinositol 3-phosphate and associates with the pollen tube endomembrane system. Plant Physiol 149:791–802
Lewis D, Crowe L (1958) Unilateral interspecific incompatibility in flowering plants. Heredity 12:233–256
Li W, Royer S, Chetelat RT (2010) Fine mapping of ui6.1, a gametophytic factor controlling pollen-side unilateral incompatibility in interspecific Solanum hybrids. Genetics. doi:genetics.110.116343
Liedl BE, McCormick S, Mutschler MA (1996) Unilateral incongruity in crosses involving Lycopersicon pennellii and L. esculentum is distinct from self-incompatibility in expression, timing and location. Sexual Plant Reprod 9:299–308
Linsley EG, Rick CM, Stephens SG (1966) Observations on the floral relationships of the Galapagos carpenter bee (Hymenoptera: Apidae). Pan-Pacific Entomol 42:1–18
Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH (2008) The strength and genetic basis of reproductive isolating barriers in flowering plants. Philos Trans Royal Soc B Biol Sci 363:3009–3021
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen Y-J, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer MLI, Jarvie TP, Jirage KB, Kim J-B, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM (2005) Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380
Martin FW (1961) The inheritance of self-incompatibility in hybrids of Lycopersicon Esculentum Mill. x L. Chilense Dun. Genetics 46:1443–1454
Martin FW (1964) The inheritance of unilateral incompatibility in Lycopersicon hirsutum. Genetics 50:459–469
Matton DP, Maes O, Laublin G, Xike Q, Bertrand C, Morse D, Cappadocia M (1997) Hypervariable domains of self-incompatibility RNases mediate allele-specific pollen recognition. Plant Cell 9:1757–1766
McClure B, Mou B, Canevascini S, Bernatzky R (1999) A small asparagine-rich protein required for S-allele-specific pollen rejection in Nicotiana. Proc Natl Acad Sci USA 96:13548–13553
McCormick S (1991) Transformation of tomato with Agrobacterium tumefaciens. Plant Tissue Cult Manual B6:1–9
McGuire DC, Rick CM (1954) Self-Incompatibility in species of Lycopersicon sect. Eriopersicon and hybrids with L. esculentum. Hilgardia 23:101–124
Moses M (1968) Synaptinemal complex. Ann Rev Genet 2:363–412
Moses M, Poorman P (1984) Synapsis, synaptic adjustment and DNA synthesis in mouse oocytes. Chromosom Today 8:99–103
Moyle LC, Graham EB (2005) Genetics of hybrid incompatibility between Lycopersicon esculentum and L. hirsutum. Genetics 169:355–373
Moyle LC, Nakazato T (2008) Comparative genetics of hybrid incompatibility: sterility in two Solanum species crosses. Genetics 179:1437–1453
Moyle LC, Nakazato T (2009) Complex epistasis for Dobzhansky-Muller hybrid incompatibility in Solanum. Genetics 181:347–351
Moyle LC, Nakazato T (2010) Hybrid incompatibility “snowballs” between Solanum species. Science 329:1521–1523
Mueller HJ (1942) Isolating mechanisms, evolution and temperature. Biol Symp 6:71–125
Murfett J, McClure BA (1998) Expressing foreign genes in the pistil: a comparison of S-RNase constructs in different Nicotiana backgrounds. Plant Mol Biol 37:561–569
Murfett J, Cornish EC, Ebert PR, Bonig I, McClure BA, Clarke AE (1992) Expression of a self-incompatibility glycoprotein (S2-Ribonuclease) from Nicotiana alata in Transgenic Nicotiana tabacum. Plant Cell 4:1063–1074
Murfett J, Atherton TL, Mou B, Gasser CS, McClure BA (1994) S-RNase expressed in transgenic Nicotiana causes S-allele-specific pollen rejection. Nature 367:563–566
Murfett J, Strabala TJ, Zurek DM, Mou B, Beecher B, McClure BA (1996) S-RNase and interspecific pollen rejection in the Genus Nicotiana: multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell 8:943–958
Mutschler M, Liedl B (1994) Interspecific crossing barriers in Lycopersicon and their relationship to self-incompatibility. In: Williams E (ed) Genetic control of self-incompatibility and reproductive development in flowering plants. Kluwer Academic, Netherlands, pp 164–188
Nasrallah JB (2002) Recognition and rejection of self in plant reproduction. Science 296:305–308
Nasrallah JB, Liu P, Sherman-Broyles S, Schmidt R, Nasrallah ME (2007) Epigenetic mechanisms for breakdown of self-incompatibility in interspecific hybrids. Genetics 175:1965–1973
Nesbitt TC, Tanksley SD (2002) Comparative sequencing in the genus Lycopersicon. Implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics 162:365–379
Noor MAF, Bertucci LA, Reiland J (2001) Chromosomal inversions and the reproductive isolation of species. PNAS USA 98:12084–12088
O’Brien M, Kapfer C, Major G, Laurin M, Bertrand C, Kondo K, Kowyama Y, Matton DP (2002) Molecular analysis of the stylar-expressed Solanum chacoense small asparagine-rich protein family related to the HT modifier of gametophytic self-incompatibility in Nicotiana. Plant J 32:985–996
Peralta IE, Spooner DM, Knapp S (2008) Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae). Syst Botany Monogr 84:186
Qiao H, Wang H, Zhao L, Zhou J, Huang J, Zhang Y, Xue Y (2004) The F-box protein AhSLFS2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell 16:582–595
Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5:e1000621
Quiros C (1991) Lycopersicon cytogenetics. In: Tsuchiya T, Gupta PK (eds) Chromosome engineering in plants: genetics, breeding, evolution (Part B). Elsevier Science Publishers, Amsterdam, pp 119–137
Rick CM (1950) Pollination relations of Lycopersicon esculentum in native and foreign regions. Evolution 4:110–122
Rick MC (1988) Tomato-like nightshades: affinities, autoecology, and breeders’ opportunities. Econ Bot 42:145–154
Rick CM, Chetelat RT (1991) The breakdown of self-incompatibility in Lycopersicon hirsutum. In: Hawkes L, Nee E (eds) Solanaceae III: taxonomy, chemistry, evolution, Royal Botanical Gardens Kew and Linnean Society of London, pp 253–256
Rick CM, Tanksley SD (1981) Genetic variation in Solanum pennellii: comparisons with two other sympatric tomato species. Plant Syst Evol 139:11–45
Rick CM, Holle M, Robbin TW (1978) Rates of cross-pollination in Lycopersicon pimpinellifolium: impact of genetic variation in floral characters. Plant Syst Evol 129:31–44
Rick CM, Fobes JF, Tanksley SD (1979) Evolution of mating systems in Lycopersicon hirsutum as deduced from genetic variation in electrophoretic and morphological characters. Plant Syst Evol 132:279–298
Rieseberg LH, Willis JH (2007) Plant speciation. Science 317:910–914
Rieseberg LH, Linder CR, Seiler GJ (1995) Chromosomal and genic barriers to introgression in helianthus. Genetics 141:1163–1171
Rieseberg LH, Whitton J, Gardner K (1999) Hybrid zones and the genetic architecture of a barrier to gene flow between Two sunflower species. Genetics 152:713–727
Rodriguez F, Wu F, Ane C, Tanksley S, Spooner D (2009) Do potatoes and tomatoes have a single evolutionary history, and what proportion of the genome supports this history? BMC Evol Biology 9:191
Sassa H, Hirano H (2006) Identification of a new class of pistil-specific proteins of Petunia inflata that is structurally similar to, but functionally distinct from, the self-incompatibility factor HT. Mol Gen Genomics 275:97–104
Schopfer CR, Nasrallah ME, Nasrallah JB (1999) The male determinant of self-incompatibility in Brassica. Science 286:1697–1700
Sheoran IS, Ross AR, Olson DJ, Sawhney VK (2007) Proteomic analysis of tomato (Lycopersicon esculentum) pollen. J Exp Bot 58:3525–3535
Sherman JD, Stack SM (1992) Two-dimensional spreads of synaptonemal complexes from solanaceous plants. Genome 35:354–359
Sherman JD, Stack SM (1995) Two-dimensional spreads of synaptonemal complexes from solanaceous plants. VI. High-resolution recombination nodule map for tomato (Lycopersicon esculentum). Genetics 141:686–708
Stack SM (1982) Two-dimensional spreads of synaptonemal complexes from solanaceous plants. I. The technique. Stain Technol 57:265–272
Stack SM, Anderson LK (1986a) Two-dimensional spreads of synaptonemal complexes from solanaceous plants. II. Synapsis in Lycopersicon esculentum. Am J Botany 73:264–281
Stack SM, Anderson LK (1986b) Two-dimensional spreads of synaptonemal complexes from solanaceous plants. III. Recombination nodules and crossing over in Lycopersicon esculentum (tomato). Chromosoma 94:253–258
Stack SM, Anderson LK (2009) Electron microscopic immunogold localization of recombination-related proteins in spreads of synaptonemal complexes from tomato microsporocytes. In: Keeney S (ed) Meiosis. Humana Press, Inc, Totowa, pp 147–169
Stack SM, Royer SM, Shearer LA, Chang SB, Giovannoni JJ, Westfall DH, White RA, Anderson LK (2009) Role of fluorescence in situ hybridization in sequencing the tomato genome. Cytogenet Genome Res 124:339–350
Swanson C (1957) Cytology and cytogenetics. Printice-Hall, Inc), Englewood Cliffs
Szinay D (2010) The development of FISH tools for genetic, phylogenetic and breeding studies in tomato (Solanum lycopersicum). Wageningen University, The Netherlands
Torres C (2000) Pollen size evolution: correlation between pollen volume and pistil length in Asteraceae. Sex Plant Reprod 12:365–370
von Wagenheim K-H (1957) Das Pachytan und der weiter Ablauf der meiose in diploiden Solanum-Arten und–Bastarden. Chromosoma 8:671–690
von Wettstein D, Rasmussen SW, Holm PB (1984) The synaptonemal complex in genetic segregation. Ann Rev Genet 18:331–413
Widmer A, Lexer C, Cozzolino S (2008) Evolution of reproductive isolation in plants. Heredity 102:31–38
Williams EG, Rouse JL (1990) Relationships of pollen size, pistil length and pollen tube growth rates in Rhododendron and their influence on hybridization. Sex Plant Reprod 3:7–17
Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392:818–821
Yamane H, Ikeda K, Ushijima K, Sassa H, Tao R (2003) A pollen-expressed gene for a novel protein with an F-box motif that is very tightly linked to a gene for S-RNase in two species of cherry, Prunus cerasus and P. avium. Plant Cell Physiol 44:764–769
Acknowledgments
This work was supported by the National Science Foundation, grant number DBI-0605200. We thank Ms. Ashley Denney and Ms. Margaret Fleming for helpful editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Scott Russell.
Rights and permissions
About this article
Cite this article
Bedinger, P.A., Chetelat, R.T., McClure, B. et al. Interspecific reproductive barriers in the tomato clade: opportunities to decipher mechanisms of reproductive isolation. Sex Plant Reprod 24, 171–187 (2011). https://doi.org/10.1007/s00497-010-0155-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-010-0155-7