Abstract
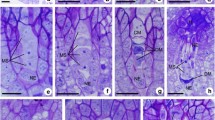
Gametophytic apomixis is an asexual mode of reproduction by seeds. This trait is present in several plant families and is strongly associated with polyploidy. Paspalum rufum is a forage grass with sexual self-incompatible diploids (2n = 2x = 20) and aposporous-apomictic pseudogamous tetraploids (2n = 4x = 40). In previous work embryological observations of the diploid genotype Q3754 showed 8.8–26.8% of the ovaries having one meiotic plus an aposporous-like embryo sac, suggesting some capability for apomictic reproduction. The objective of this work was to characterize progenies derived from Q3754 to determine if aposporous sacs were functional and generated progenies via apomixis at the diploid level. Re-examination of Q3754 ovaries showed that 12.5% of them contained one sexual plus an aposporous sac confirming previous results. Progeny tests were carried out on two experimental families (H1 and S1) employing heterozygous RAPD marker loci. Family H1 was obtained crossing Q3754 with a natural diploid genotype (Q3861) and S1 derived from the induced self-pollination of Q3754. Genetic analysis of H1 showed that all individuals derived from sexual reproduction. However, 5 out of 95 plants from S1 showed the same heterozygous state as the mother plant for 14 RAPD loci suggesting a clonal origin. Further experiments, designed to test the functionality of aposporous sacs by flow cytometric analyses, were carried out on a third family (M1) obtained by crossing Q3754 with the tetraploid plant Q3785. Histograms of 20 M1 plants showed 15 diploids (75%), 4 triploids (20%) and 1 tetraploid (5%). Triploids and the tetraploid may have originated from functional aposporous embryo sacs. Likewise, the reconstruction of the developmental route of 40 individual seeds demonstrated that 11 of them (27.5%) derived from fertilized aposporic sacs. The results presented in this work indicate that gametophytic apomixis is effectively expressed at the diploid level in Paspalum rufum and could be the foundation of a recurrent auto-polyploidization process in the species.



Similar content being viewed by others
References
Asker S (1970) Apomixis and sexuality in the Potentilla argentea complex. II Crosses within the complex. Hereditas 66:189–204
Asker SE, Jerling L (1992) Apomixis in plants. CRC, Boca Raton
Bicknell RA, Lambie SC, Butler RC (2003) Quantification of progeny classes in two facultatively apomictic accessions of Hieracium. Hereditas 138:11–20
Böcher TW (1951) Cytological and embryological studies in the amphi-apomictic Arabis holboellii complex. Kongel Danske Vidensk Selskab Biol Skr 6:159
Burson BL (1992) Cytology and reproductive behavior of hybrids between Paspalum urvillei and two hexaploid Paspalum dilatatum biotypes. Genome 35(6):1002–1006
Burton GW, Hanna WW (1986) Bahiagrass tetraploids produced by making (apomictic tetraploid × diploid) × diploid hybrids. Crop Sci 26:1254–1256
Burton GW, Hanna WW (1992) Using apomictic tetraploids to make a self-incompatible diploid Pensacola bahiagrass clone set seed. J Hered 83:305–306
Darlington CD (1939) Evolution of genetic systems. Cambridge University Press, Cambridge
Daurelio DL, Espinoza F, Quarin CL, Pessino SC (2004) Genetic diversity in sexual diploid and apomictic tetraploid populations of Paspalum notatum situated in sympatry or allopatry. Plant Syst Evol 244:189–199
de Wet JMJ, Stalker HT (1974) Gametophytic apomixis and evolution in plants. Taxon 23:689–697
Espinoza F, Pessino SC, Quarin CL, Valle EM (2002) Effect of pollination timing on the rate of apomictic reproduction revealed by RAPD markers. Ann Bot 89:165–170
Harlan JR, Brooks MH, Borgaonkar DS, de Wet JMJ (1964) Nature and inheritance of apomixis in Bothriochloa and Dichanthium. Bot Gaz 125:41–46
Holm S, Ghatnekar L (1996) Sexuality and no apomixis found in crossing experiments with diploid Potentilla argentea. Hereditas 125:77–82
Holm S, Ghatnekar L, Bengtsson BO (1997) Selfing and outcrossing but no apomixis in two natural populations of diploid Potentilla argentea. J Evol Biol 10:343–352
Kantama L, Lambert Y, Hu H, de Jong H, de Vries SC, Russinova E (2006) Use of the SSLP-based method for detection of rare apomictic events in a sexual AtSERK1 transgenic Arabidopsis population. Sex Plant Reprod 19:73–82
Kantama L, Sharbel TF, Schranz ME, Mitchell-Olds T, de Vries S, de Jong H (2007) Diploid apomicts of the Boechera holboellii complex display large-scale chromosome substitutions and aberrant chromosomes. Proc Natl Acad Sci USA 104:14026–14031
Marshall DR, Brown AHD (1974) Estimation of the level of apomixis in plant populations. Heredity 32:321–333
Martínez EJ, Hopp E, Stein J, Ortiz JPA, Quarin CL (2003) Genetic characterization of apospory in tetraploid Paspalum notatum based on the identification of linked molecular markers. Mol Breed 12:319–327
Mather K (1951) The measurement of linkage in heredity, 2nd edn. Methuen and Co., London
Matzk F, Meister A, Schubert I (2000) An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J 21:97–108
Müntzing A, Müntzing G (1945) The mode of reproduction of hybrids between sexual and apomictic Potentilla argentea. Bot Notiser 98:49–71
Naumova TN, Hayward MD, Wagenvoort M (1999) Apomixis and sexuality in diploid and tetraploid accessions of Brachiaria decumbens. Sex Plant Reprod 12:43–52
Nogler GA (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 475–518
Norrmann GA, Quarin CL, Burson BL (1989) Cytogenetics and reproductive behavior of different chromosome races in six Paspalum species. J Hered 80:24–28
Norrmann GA, Bovo OA, Quarin CL (1994) Post-zygotic seed abortion in sexual diploid × apomictic tetraploid intraspecific Paspalum crosses. Aust J Bot 42:449–456
Ortiz JPA, Pessino SC, Leblanc O, Hayward MD, Quarin CL (1997) Genetic fingerprinting for determining the mode of reproduction in Paspalum notatum, a subtropical apomictic forage grass. Theor Appl Genet 95:850–856
Ortiz JPA, Pessino SC, Bhat V, Hayward MD, Quarin CL (2001) A genetic linkage map of diploid Paspalum notatum. Crop Sci 41:823–830
Pupilli F, Caceres ME, Quarín CL, Arcioni S (1997) Segregation analysis of RFLP markers reveals a tetrasomic inheritance in apomictic Paspalum simplex. Genome 40:822–828
Quarin CL (1986) Seasonal changes in the incidence of apomixis of diploid and tetraploid plants of Paspalum cromyrrhizon. Euphytica 35:515–522
Quarin CL (1992) The nature of apomixis and its origin in panicoid grasses. Apomixis Newsl 5:8–15
Quarin CL (1999) Effect of pollen source and pollen ploidy on endosperm formation and seed set in pseudogamous apomictic Paspalum notatum. Sex Plant Reprod 11:331–335
Quarin CL, Lombardo EP (1986) Niveles de ploidía y distribución geográfica de Paspalum quadrifarium (Gramineae). Mendeliana 7:101–107
Quarin CL, Norrmann GA (1987) Cytology and reproductive behavior of Paspalum equitans, P. ionanthum and their hybrids with diploid and tetraploid cytotypes of P. cromyorrhizon. Bot Gaz 148:386–391
Quarin CL, Norrmann GA, Urbani MH (1989) Polyploidization in aposporous Paspalum species. Apomixis Newsl 1:28–29
Quarin CL, Norrmann GA, Espinoza F (1998) Evidence for autoploidy in apomictic Paspalum rufum. Hereditas 129:119–124
Quarin CL, Espinoza F, Martínez EJ, Pessino SC, Bovo OA (2001) A rise of ploidy level induces the expression of apomixis in Paspalum notatum. Sex Plant Reprod 13:243–249
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Savidan Y (2000) Apomixis: genetics and breeding. In: Janick J (ed) Plant breeding reviews, vol 18. Wiley, London, pp 13–86
Soltis DE, Soltis PS (1999) Polyploidy: recurrent formation and genome evolution. Trends Ecol Evol 14:348–352
Stam P (1993) Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J 3:739–744
Stein J, Quarin CL, Martínez EJ, Pessino SC, Ortiz JPA (2004) Tetraploid races of Paspalum notatum show polysomic inheritance and preferential chromosome pairing around the apospory-controlling locus. Theor Appl Genet 109:186–191
Urbani MH, Quarin CL, Espinoza F, Penteado MIO, Rodríguez IF (2002) Cytogeography and reproduction of the Paspalum simplex polyploid complex. Plant Syst Evol 236:99–105
Yamauchi A, Hosokawa A, Nagata H, Shimoda M (2004) Triploid bridge and role of parthenogenesis in evolution of autopolyploidy. Am Nat 164:101–111
Zuloaga FO, Morrone O (2005) Revisión de las especies de Paspalum para América del Sur Austral (Argentina, Bolivia, Sur de Brasil, Chile, Paraguay y Uruguay). In: Hollowell VC (ed). Missouri Botanical Garden Press, St Luis
Acknowledgments
The authors wish to thank Dr Silvina Pessino and Prof. Michael Hayward for critically reading the manuscript. They also thank Florencia Galdeano for her technical assistance. This study was financed by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina, PICT 2003 No. 13578 and PAV 2003 No. 137/3; Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina, PIP 2004 No. 6805. Centro Argentino Brasilero de Biotecnología (CABBIO 2004 No. 012). L. Siena and M. Sartor received fellowships from CONICET and F. Espinoza, C.L. Quarin and J.P.A. Ortiz are career members of CONICET.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.S. Heslop-Harrison.
Rights and permissions
About this article
Cite this article
Siena, L.A., Sartor, M.E., Espinoza, F. et al. Genetic and embryological evidences of apomixis at the diploid level in Paspalum rufum support recurrent auto-polyploidization in the species. Sex Plant Reprod 21, 205–215 (2008). https://doi.org/10.1007/s00497-008-0080-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-008-0080-1