Abstract
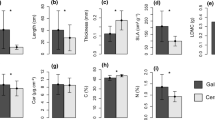
The ecology of forest and savanna trees species will largely determine the structure and dynamics of the forest–savanna boundaries, but little is known about the constraints to leaf trait variation imposed by selective forces and evolutionary history during the process of savanna invasion by forest species. We compared seasonal patterns in leaf traits related to leaf structure, carbon assimilation, water, and nutrient relations in 10 congeneric species pairs, each containing one savanna species and one forest species. All individuals were growing in dystrophic oxisols in a fire-protected savanna of Central Brazil. We tested the hypothesis that forest species would be more constrained by seasonal drought and nutrient-poor soils than their savanna congeners. We also hypothesized that habitat, rather than phylogeny, would explain more of the interspecific variance in leaf traits of the studied species. We found that throughout the year forest trees had higher specific leaf area (SLA) but lower integrated water use efficiency than savanna trees. Forest and savanna species maintained similar values of predawn and midday leaf water potential along the year. Lower values were measured in the dry season. However, this was achieved by a stronger regulation of stomatal conductance and of CO2 assimilation on an area basis (A area) in forest trees, particularly toward the end of the dry season. Relative to savanna trees, forest trees maintained similar (P, K, Ca, and Mg) or slightly higher (N) leaf nutrient concentrations. For the majority of traits, more variance was explained by phylogeny, than by habitat of origin, with the exception of SLA, leaf N concentration, and A area, which were apparently subjected to different selective pressures in the savanna and forest environments. In conclusion, water shortage during extended droughts would be more limiting for forest trees than nutrient-poor soils.






Similar content being viewed by others
References
Adams HD, Guardiola-Claramonte M, Barron-Gafford G, Villegas JC, Breshears DD, Zou CB, Troch PA, Huxman TE (2009) Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. PNAS 106:7063–7066
Aerts R, Chapin FS III (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications, Oxford
Araújo JF, Haridasan M (2007) Relação entre deciduidade e concentrações foliares de nutriente em espécies lenhosas do Cerrado. Rev Bras Bot 30:533–542
Barros FV, Goulart MF, Teles SBS, Lovato MB, Valladares F, Lemos Filho JP (2011) Phenotypic plasticity to light of two congeneric trees from contrasting habitats: Brazilian Atlantic Forest versus Cerrado (savanna). Plant Biol 14:208–215
Bond W (2010) Do nutrient-poor soils inhibit development of forests? A nutrient stock analysis. Plant Soil 334:334–347
Bond WJ, Midgley JJ (2000) Ecology of sprouting in woody plants: the persistence niche. Trends Ecol Evo 16:45–51
Borchert R (1998) Responses of tropical trees to rainfall seasonality and its long-term changes. Clim Chang 39:381–393
Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Campanello P, Scholz F (2005) Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in Neotropical savanna trees. Trees 19:296–304
Caldas LS, Bravo C, Piccolo H, Faria CRSM (1992) Measurement of leaf area with a hand-scanner linked to a microcomputer. Revista Brasileira de Fisiologia Vegetal 4:17–20
Cole MM (1992) Influence of physical factor on the nature and dynamics of forest–savanna boundaries. In: Furley PA, Proctor J, Ratter JA (eds) Nature and dynamics of forest-savanna boundaries. Chapman and Hall, London, pp 63–75
Eamus D, Myers B, Duff G, Willians R (1999) Seasonal changes in photosynthesis of eight savanna tree species. Tree Phys 19:665–671
Ehleringer JR, Hall AE, Farquhar GD (1993) Stable isotopes an plant carbon–water relations. Academic Press, San Diego
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Phys 11:539–552
Felfili JM, Silva Júnior MC (1993) A comparative study of Cerrado (sensu stricto) vegetation in Central Brazil. J Trop Ecol 9:277–289
Franco AC (2002) Ecophysiology of woody plants. In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 178–197
Franco AC, Lüttge U (2002) Midday depression in savanna trees: coordinated adjustments in photochemical, efficiency, photorespiration, CO2 assimilation and water use efficiency. Oecologia 131:356–365
Franco AC, Bustamante M, Caldas LS, Goldstein G, Meinzer FC, Kozovits AR, Rundel P, Coradin VTR (2005) Leaf functional traits of neotropical savanna trees in relation to seasonal water deficit. Trees 19:326–335
Gotsch S, Geiger EL, Franco AC, Goldstein G, Meinzer FC, Hoffmann WA (2010) Allocation to leaf area and sapwood area affects water relations of co-occurring savanna and forest trees. Oecologia 163:291–301
Güsewell S (2004) N:P ratios in terrestrial plants: variations and functional significance. New Phytol 164:243–266
Hao GY, Hoffmann WA, Scholtz FG, Bucci SJ, Meinzer FC, Franco AC, Cao KF, Goldstein G (2008) Stem and leaf hydraulics of congeneric tree species from adjacent tropical savanna and forest ecosystems. Oecologia 155:405–415
Haridasan M (1998) Solos de mata de galeria e nutrição mineral de espécies arbóreas em condições naturais. In: Ribeiro JF (ed) Cerrado: Matas de Galeria. Embrapa-CPAC, Planaltina, p 19–28
Haridasan M (2008) Nutritional adaptations of native plants of the Cerrado biome in acid soils. Braz J Plant Physiol 20(3):183–195
Hennenberg KJ, Fischer F, Kouadio K, Goetze D, Orthmann B, Linsenmair KE, Jeltsch F, Porembski S (2006) Phytomass and fire occurrence along forest–savanna transects in the Comoé National Park, Ivory Coast. J Trop Ecol 22:303–311
Hoffmann WA, Franco AC (2008) The importance of evolutionary history in studies of plant physiological ecology: examples from Cerrados and forests of Central Brazil. Braz J Plant Phys 20:247–256
Hoffmann WA, Schroeder W, Jackson RB (2002) Positive feedbacks of fire, climate, and vegetation change and the conversion of tropical savannas. Geophys Res Method 15:2052
Hoffmann WA, Orthen B, Franco AC (2004) Constraints to seedling success of savanna and forest trees across the savanna–forest boundary. Oecologia 140:252–260
Hoffmann WA, Franco AC, Moreira MZ, Haridasan M (2005a) Specific leaf area explains differences in leaf traits between congeneric savanna and forest trees. Funct Ecol 19:932–940
Hoffmann WA, da Silva ER, Machado GC, Bucci SJ, Scholz FG, Goldstein G, Meinzer FC (2005b) Seasonal leaf dynamics across a tree density gradient in a Brazilian savanna. Oecologia 145:307–316
Hoffmann WA, Adasme R, Haridasan M, Carvalho MT, Geiger E, Pereira MAB, Gotsch SB, Franco AC (2009) Tree topkill, not mortality, governs the dynamics of savanna–forest boundaries under frequent fire in Central Brazil. Ecology 90:1326–1337
Hoffmann WA, Geiger EL, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, Haridasan M, Franco AC (2012a) Ecological thresholds at the savanna–forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol Lett 15:759–768
Hoffmann WA, Jaconis SY, Geiger EL, Gotsch SG, Franco AC (2012b) Fuels or microclimate? Understanding the drivers of fire feedbacks at savanna–forest boundaries. Aust Ecol 37:634–643
Meinzer FC, Goldstein G, Franco AC, Bustamante M, Igler E, Jackson P, Caldas LS, Rundel PW (1999) Atmospheric and hydraulic limitations on transpiration in Brazilian Cerrado woody species. Funct Ecol 13:273–282
Mendonça RC, Felfili JM, Walter BMT, Silva-Júnior MC, Rezende AB, Filgueiras TS, Nogueira PE, Fagg CW (2008) Flora Vascular do Bioma Cerrado: checklist com 12.356 espécies. In: Sano SM, Almeida SP, Ribeiro JF (org.) Cerrado: Ecologia e Flora, vol. 2. Embrapa Cerrados, Brasília, p 213–228
Moreira A (2008) Effects of fire protection on savanna structure in Central Brazil. J Biogeogr 27:1021–1029
Oliveira-Filho AT, Ratter JA (2002) Vegetation physiognomies and woody flora of the Cerrado biome. In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 121–139
Prado CHBA, Wenhui Z, Rojas MHC, Souza GM (2004) Seasonal leaf gas exchange and water potential in a woody Cerrado species community. Braz J Plant Phys 16:7–16
Prior LD, Bowman DMJS, Eamus D (2004a) Seasonal differences in leaf attributes in Australian tropical tree species: family and habitat comparisons. Funct Ecol 18:707–718
Prior LD, Eamus D, Bowman DMJS (2004b) Tree growth rates in north Australian savanna habitats: seasonal patterns and correlations with leaf attributes. Aust J Bot 52:303–314
Ribeiro JF, Walter BMT (2001) As matas de galeria no contexto do bioma Cerrado. In: Ribeiro JF, Fonseca CEL, Sousa-Silva JC (eds) Cerrado: caracterização e recuperação de Matas de Galeria. Embrapa Cerrados, Planaltina, pp 29–47
Ribeiro JF, Walter BMT (2008) Fitofisionomias do bioma Cerrado. In: Sano S, Almeida SP, Ribeiro JF (eds) Cerrado: Ecologia e flora. Embrapa Cerrados, Planaltina, pp 19–45
Rojas-Jiménez K, Holbrook NM, Gutiérrez-Soto MV (2007) Dry-season leaf flushing of Enterolobium cyclocarpum (ear-pod tree): above- and belowground phenology and water relations. Tree Phys 27:1561–1568
Rosenthal R, Rosnow RL (1985) Contrast analysis: focused comparisons in the analysis of variance. Cambridge University Press, New York
Rossatto DR, Hoffmann WA, Franco AC (2009) Differences in growth patterns between co-occurring forest and savanna affect the forest savanna boundary. Funct Ecol 23:689–698
Rossatto DR, Silva LCR, Villalobos-Vega R, Sternberg LSL, Franco AC (2012) Depth of water uptake in woody plants relates to groundwater level and vegetation structure along a topographic gradient in a neotropical savanna. Env Exp Bot 77:259–266
Silva-Júnior MC, Felfili JM, Nogueira PE, Rezende AV (1998) Análise florística das Matas de Galeria do Distrito Federal. In: Ribeiro JF (ed) Cerrado: Matas de Galeria. Embrapa-CPAC, Planaltina, pp 53–84
Simon MF, Pennington T (2012) Evidence for adaptation to fire regimes in the tropical savannas of the Brazilian Cerrado. Int J Plant Sci 173:711–723
Staver AC, Archibald SA, Levin SA (2011) Tree cover in sub-Saharan Africa: rainfall and fire constrain forest and savanna as alternative stable states. Ecology 92:1063–1072
Valladares F, Niinemets U (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Eco Syst 39:237–257
van Mantgem PJ, Stephenson NL, Byrne JC, Daniels LD, Franklin JF, Fule PZ, Harmon ME, Larson AJ, Smith JM, Taylor AH, Veblen T (2009) Widespread increase of tree mortality rates in the western United States. Science 323:521–524
Veenendaal EM, Swaine MD, Agyeman VK et al (1995) Differences in plant and soil water relations in and around a forest gap in West Africa during the dry season may influence seedling establishment and survival. J Ecol 83:83–90
Veneklass EJ, Fajardo A, Obregon S, Lozano J (2005) Gallery forest types and their environmental correlates in a Colombian savanna landscape. Ecography 28:236–252
Vincens A, Garcin Y, Buchet G (2007) Influence of rainfall seasonality on African lowland vegetation during the Late Quaternary: pollen evidence from Lake Masoko, Tanzania. J Biogeogr 34:1274–1288
Warton DI, Wright IJ, Falster DS, Westoby D (2006) Bivariate line-fitting methods for allometry. Biol Rev 81:269–291
Westoby M (1998) A leaf-height-seed (LHS) plant strategy scheme. Plant Soil 199:213–227
Zar JH (1999) Biostatistical analysis. Prentice Hall, New Jersey
Zhang JL, Zhu JJ, Cao KF (2007) Seasonal variation in photosynthesis in six woody species with different leaf phenology in a valley savanna in southwestern China. Trees 21:631–643
Acknowledgments
This work was funded by National Science Foundation (No. DEB-0542912, USA), Mellon Foundation (USA), CNPq (Brazil) and CAPES (Brazil).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Matyssek.
Rights and permissions
About this article
Cite this article
Rossatto, D.R., Hoffmann, W.A., de Carvalho Ramos Silva, L. et al. Seasonal variation in leaf traits between congeneric savanna and forest trees in Central Brazil: implications for forest expansion into savanna. Trees 27, 1139–1150 (2013). https://doi.org/10.1007/s00468-013-0864-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-013-0864-2