Abstract
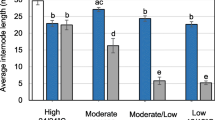
Loss of apical dominance in boron-deficient trees has been suggested to be due to frost damage of terminal buds and leaders. Excessive nitrogen (N) supply can exacerbate boron (B) deficiency by the dilution-effect. N may also have direct effects on winter hardiness. We studied frost hardening of buds of Norway spruce (Picea abies L. Karst.) in healthy-looking trees and in trees with growth disturbances. The effect of B and N on frost hardiness was studied in a factorial fertilisation experiment during cold acclimation. Frost hardiness was determined by differential temperature analysis (DTA) and scoring of visual damage. In a DTA profile of apical buds with a piece of stem, low-temperature exotherm (LTE) predicted bud injury, while two of the observed high-temperature exotherms and two of the observed intermediate-temperature exotherms were non injurious. Appearance of LTE followed changes in air temperature. The risk of frost damage was not affected by fertilisation treatments or previously observed growth disturbances. However, when the bud structure was deformed by severe B deficiency, the supercooling ability disappeared. Such buds are probably killed by freezing in nature and therefore, frost damage may play a secondary role in the development of growth disturbances.





Similar content being viewed by others
References
Aronsson A (1980) Frost hardiness in Scots pine (Pinus sylvestris L.) - Hardiness during winter and spring in young trees of different mineral nutrient status. Stud For Suecica 155:1–27
Aronsson A (1983) Growth disturbances caused by boron deficiency in some fertilized pine and spruce stands on mineral soils. Commun Inst For Fenniae 116:116–122
Ashworth EN, Abeles FB (1984) Freezing behaviour of water in small pores and the possible role in the freezing of plant tissues. Plant Physiol 76:201–204
Atkins PW (1986) Physical chemistry, 3rd edn. Oxford University Press, Oxford
Badulescu Valle RD (2002) Mechanisms of frost adaptation and freeze damage in grapevine buds. Academic Dissertation, University of Hohenheim; http://www.opus-ho.uni-stuttgart.de/hop/volltexte/2003/25/, pp 1–115
Bigras FJ, Gonzales A, D’Aoust AL, Hebert C (1996) Frost hardiness, bud phenology and growth of containerized Picea mariana seedlings grown at three nitrogen levels and three temperature regimes. New For 12:243–259
Bilkova J, Albrechtova J, Opatrna J (1999) Histochemical detection and image analysis of non-specific esterase activity and the amount of polyphenols during annual bud development in Norway spruce. J Exp Bot 35:1129–1138
Braekke FH (1979) Boron deficiency in forest plantations on peatland in Norway. Rep Norwegian For Res Inst 35(3):217–236
Brown PH, Shelp BJ (1997) Boron mobility in plants. Plant Soil 193:82–101
Burr KE, Hawkins CDB, L’Hirondelle SJL, Binder WD, George MF, Repo T (2001) Methods for measuring cold hardiness of conifers. In: Bigras FJ, Colombo SJ (eds) Conifer cold hardiness. KluwerAcademic, Dordrecht, The Netherlands, pp 369–401.
Cajander AK (1949) Forest site types and their significance. Acta For Fennica 56:21–28
Dannel F, Pfeffer H, Römheld V (1998) Compartmentation of boron in roots and leaves of sunflower as affected by boron supply. J Plant Physiol 153:615–622
DeHayes DH, Ingle MA, Waite CE (1989) Nitrogen fertilization enhances cold tolerance of red spruce. Canadian J For Res 19:1037–1043
Ferm A, Hytönen J, Lähdesmäki P, Pietiläinen P (1990) Effects of high nitrogen deposition on forests: Case studies close to fur animal farms. In: Kauppi P, Anttila P, Kenttämies K (eds) Acidification in Finland. Springer-Verlag, Berlin, pp 635–668
Fleischer A, O’Neill MA, Ehwald R (1999) The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalactouronan II. Plant Physiol 121:829–838
George MF, Burke MJ, Pellet HM, Johnson AG (1974) Low-temperature exotherms and woody plant distribution. Hortscience 9:519–522
Gusta LV, Tyler NJ, Chen THH (1983) Deep undercooling in woody taxa growing north of the −40°C isotherm. Plant Physiol 72:122–128
Halonen O, Tulkki H, Derome J (1983) Nutrient analysis methods. The Finnish Forest Research Institute. Research Papers 121:1–28
Hellergren J (1981) Frost hardiness development in Pinus silvestris seedlings in response to fertilization. Physiol Planta 52:297–301
Hu H, Brown PJ (1994) Localization of boron in cell walls of squash and tobacco and its association with pectin. Plant Physiol 105:681–689
Ishikawa M, Price WS, Ide H, Arata Y (1997) Visualization of freezing behaviours in leaf and flower buds of full-moon maple by nuclear magnetic resonance microscopy. Plant Physiol 115:1515–1524
Jalkanen R (1990) Nitrogen fertilization as a cause of dieback of Scots pine at Paltamo, northern Finland. Aquilo Ser Bot 29:25–31
Jones KS, MacKersie BD, Paroschy J (2000) Prevention of ice propagation by permeability barriers in bud axes of Vitis vinifera. Can J Bot 78:3–9
Jönsson AM, Ingerslev M, Raulund-Rasmussen K (2004) Frost sensitivity and nutrient status in a fertilized Norway spruce stand in Denmark. Forest Ecol Manage 201:199–209
Jukka L (1988) Metsänterveysopas. Metsätuhot ja niiden torjunta, Samerka, Helsinki, 138 pp
Kaunisto S (1984) Yhteenveto lannoitustutkimuksista metsikön perustamisen ja taimikonhoidon yhteydessä turvemailla. Suo 35:116–126
Ketchie DO, Kammereck R (1987) Seasonal variation of cold resistance in Malus woody tissue as determined by differential thermal analysis and viability tests. Can J Bot 65:2640–2645
Lehto T, Lavola A, Julkunen-Tiitto R, Aphalo PJ (2004) Boron retranslocation in Scots pine and Norway spruce. Tree Physiol 24:1011–1017
L’Hirondelle SJ, Jacobson JS, Lassoie JP (1992) Acidic mist and nitrogen fertilization effects on growth, nitrate reductase activity gas exhange, and frost hardiness of red spruce seedlings. New Phytol 121:611–622
Lipavska H, Svobodova H, Albrechtova J (2000) Annual dynamics of the content of non-structural saccharides in the context of structural development of vegetative buds of Norway spruce. J Plant Physiol 157:365–373
Mälkönen E, Derome J, Kukkola M (1990) Effects of nitrogen inputs on forest ecosystems estimation based on long-term fertilisation experiments. In: Kauppi P, Anttila P, Kenttämies K (eds) Acidification in Finland. Springer-Verlag, Berlin, pp 325–347
Matoh T (1997) Boron in plant cell walls. Plant Soil 193:59–70
McNulty SG, Aber JD, Newman SD (1996) Nitrogen saturation in a high elevation New England spruce – fir stand. For Ecol Manage 84:109–121
Pietiläinen P (1984) Foliar nutrient content and 6-phosphogluconate dehydrogenase activity in vegetative buds af Scots pine on a growth disturbance area. Commun Inst For Fenniae 123:1–18
Pukacki P (1987) Deep supercooling shoot and bud tissues of Picea abies. For Ecol Manage 20:97–103
Pukacki P, Pukacka S (1987) Freezing stress and membrane injury of Norway spruce (Picea abies) tissues. Physiol Plantarum 69:156–160
Räisänen M, Repo T, Lehto T (2006) Effect of thawing time, cooling rate, and boron nutrition on freezing point of the primordial shoot in Norway spruce buds. Ann Bot, doi:10:1093/aob/mcl008
Ranta E, Rita H, Kouki J (1992) Biometria—tilastotiedettä ekologeille (in Finnish). (Biometrics—statistics for ecologists), 4th edn. Yliopistopaino, Helsinki, 569 pp
Sakai A (1979) Freezing avoidance of primordial shoots of very hardy conifer buds. Biol Sci 37:1–9
Sakai A (1982) Extraorgan freezing of primordial shoots of winter buds of conifer. In: Li PH, Sakai A (eds) Plant cold hardiness and freezing stress. Academic, New York, pp 199–209
Sakai A, Larcher W (1987) Frost survival of plants. Springer-Verlag, Berlin, 321 pp
Schaberg PG, Perkins TD, McNulty SG (1997) Effects of chronic low-level N additions on foliar elemental concentrations, morphology, and gas excange of mature montane red spruce. Can J For Res 27:1622–2629
Sutinen S, Rikala R, Lehto T (2004) Kasvuhäiriön solutason oireisto ja sen kehittyminen. (Symptoms of growth disturbance at the cell-level and the development of the disturbance). The Finnish Forest Research Institute. Research Papers 934:21–25
Tumanov II, Krasavtsev OK (1959) Zakalivanije severnyh drevesnyh rastenij otricateljnymi temperaturami (in Russian) (Hardening of woody plants by negative temperature treatment). Fiziol Rastenij 6:654–667
Wisniewski M, Davis G, Schaffer K (1991a) Mediation of deep supercooling of peach and dogwood by enzymatic modifications in cell-wall structure. Planta 184:254–260
Wisniewski M, Davis G, Arora R (1991b) Effect of macerase, oxalic acid, and EGTA on deep supercooling and pit membrane structure of xylem parenchyma of peach. Plant Physiol 96:1354–1359
Acknowledgements
We thank Rauni Oksman and Merja Essell for help in laboratory and Marja Kuskelin, Martti Vuorinen and Lauri Nykänen for help with fieldwork. This study was funded by the Academy of Finland (Decision no. 206898), Maj and Tor Nessling Foundation and the Ministry of Agriculture and Forestry in Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Beck
Rights and permissions
About this article
Cite this article
Räisänen, M., Repo, T., Rikala, R. et al. Does ice crystal formation in buds explain growth disturbances in boron-deficient Norway spruce?. Trees 20, 441–448 (2006). https://doi.org/10.1007/s00468-006-0059-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-006-0059-1