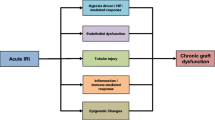
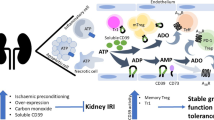
Abstract
Ischemia reperfusion (IR) injury is a process defined by the temporary loss of blood flow and tissue perfusion followed later by restoration of the same. Brief periods of IR can be tolerated with little permanent deficit, but sensitivity varies for different target cells and tissues. Ischemia reperfusion injuries have multiple causes including peripheral vascular disease and surgical interventions that disrupt soft tissue and organ perfusion as occurs in general and reconstructive surgery. Ischemia reperfusion injury is especially prominent in organ transplantation where substantial effort has been focused on protecting the transplanted organ from the consequences of IR. A number of factors mediate IR injury including the production of reactive oxygen species and inflammatory cell infiltration and activation. In the kidney, IR injury is a major cause of acute injury and secondary loss of renal function. Transplant-initiated renal IR is also a stimulus for innate and adaptive immune-mediated transplant dysfunction. The cell surface molecule CD47 negatively modulates cell and tissue responses to stress through limitation of specific homeostatic pathways and initiation of cell death pathways. Herein, a summary of the maladaptive activities of renal CD47 will be considered as well as the possible therapeutic benefit of interfering with CD47 to limit renal IR.


Similar content being viewed by others
References
Thomas DD, Heinecke JL, Ridnour LA, Cheng RY, Kesarwala AH, Switzer CH, McVicar DW, Roberts DD, Glynn S, Fukuto JM, Wink DA, Miranda KM (2015) Signaling and stress: the redox landscape in NOS2 biology. Free Radic Biol Med 87:204–225. https://doi.org/10.1016/j.freeradbiomed.2015.06.002
Powell RW, Dyess DL, Collins JN, Roberts WS, Tacchi EJ, Swafford AN Jr, Ferrara JJ, Ardell JL (1999) Regional blood flow response to hypothermia in premature, newborn, and neonatal piglets. J Pediatr Surg 34:193–198. https://doi.org/10.1016/S0022-3468(99)90255-5
Southworth R, Shattock MJ, Hearse DJ, Kelly FJ (1998) Developmental differences in superoxide production in isolated guinea-pig hearts during reperfusion. J Mol Cell Cardiol 30:1391–1399. https://doi.org/10.1006/jmcc.1998.0707
Ataka K, Chen D, Levitsky S, Jimenez E, Feinberg H (1992) Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation 86:II371–II376
Simkhovich BZ, Marjoram P, Poizat C, Kedes L, Kloner RA (2003) Age-related changes of cardiac gene expression following myocardial ischemia/reperfusion. Arch Biochem Biophys 420:268–278. https://doi.org/10.1016/j.abb.2003.06.001
Jugdutt BI, Jelani A, Palaniyappan A, Idikio H, Uweira RE, Menon V, Jugdutt CE (2010) Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model: effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation 122:341–351. https://doi.org/10.1161/CIRCULATIONAHA.110.948190
Okaya T, Blanchard J, Schuster R, Kuboki S, Husted T, Caldwell CC, Zingarelli B, Wong H, Solomkin JS, Lentsch AB (2005) Age-dependent responses to hepatic ischemia/reperfusion injury. Shock 24:421–427. https://doi.org/10.1097/01.shk.0000181282.14050.11
Selzner M, Selzner N, Chen L, Borozan I, Sun J, Xue-Zhong M, Zhang J, McGilvray ID (2009) Exaggerated up-regulation of tumor necrosis factor alpha-dependent apoptosis in the older mouse liver following reperfusion injury: targeting liver protective strategies to patient age. Liver Transpl 15:1594–1604. https://doi.org/10.1002/lt.21864
Hammers DW, Merritt EK, Matheny RW Jr, Adamo ML, Walters TJ, Estep JS, Farrar RP (2008) Functional deficits and insulin-like growth factor-I gene expression following tourniquet-induced injury of skeletal muscle in young and old rats. J Appl Physiol (1985) 105:1274–1281. https://doi.org/10.1152/japplphysiol.90418.2008
Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE (2011) Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol 31:1748–1756. https://doi.org/10.1161/ATVBAHA.111.227314
Labat-Robert J, Robert L (2014) Longevity and aging. Role of free radicals and xanthine oxidase. A review. Pathol Biol (Paris) 62:61–66. https://doi.org/10.1016/j.patbio.2014.02.009
Qiu W, Zheng L, Gu H, Chen D, Chen Y (2008) Comparison between adult and infant lung injury in a rabbit ischemia-reperfusion model. J Thorac Cardiovasc Surg 136:352–359. https://doi.org/10.1016/j.jtcvs.2008.01.014
Chenliu C, Sheng X, Dan P, Qu Y, Claydon VE, Lin E, Hove-Madsen L, Sanatani S, Tibbits GF (2016) Ischemia-reperfusion destabilizes rhythmicity in immature atrioventricular pacemakers: a predisposing factor for postoperative arrhythmias in neonate rabbits. Heart Rhythm 13:2348–2355. https://doi.org/10.1016/j.hrthm.2016.07.022
Calabrese V, Bates TE, Stella AM (2000) NO synthase and NO-dependent signal pathways in brain aging and neurodegenerative disorders: the role of oxidant/antioxidant balance. Neurochem Res 25:1315–1341. https://doi.org/10.1023/A:1007604414773
Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, Herring CM, Tan J, Lahm T, Meldrum DR (2008) VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol 295:H2308–H2314. https://doi.org/10.1152/ajpheart.00565.2008
Lan C, Song JL, Yan LN, Yang JY, Wen TF, Li B, Xu MQ (2017) Pediatric donor to adult recipients in donation after cardiac death liver transplantation: a single-center experience. Transplant Proc 49:1383–1387. https://doi.org/10.1016/j.transproceed.2017.01.088
Paparella D, Yau TM, Young E (2002) Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 21:232–244
Gill RS, Pelletier JS, LaBossiere J, Bigam DL, Cheung PY (2012) Therapeutic strategies to protect the immature newborn myocardium during resuscitation following asphyxia. Can J Physiol Pharmacol 90:689–695. https://doi.org/10.1139/y2012-041
Reeves I, Abribat T, Laramee P, Jasmin G, Brazeau P (2000) Age-related serum levels of insulin-like growth factor-I, -II and IGF-binding protein-3 following myocardial infarction. Growth Hormon IGF Res 10:78–84. https://doi.org/10.1054/ghir.2000.0143
Heinen A, Behmenburg F, Aytulun A, Dierkes M, Zerbin L, Kaisers W, Schaefer M, Meyer-Treschan T, Feit S, Bauer I, Hollmann MW, Huhn R (2018) The release of cardioprotective humoral factors after remote ischemic preconditioning in humans is age- and sex-dependent. J Transl Med 16:112. https://doi.org/10.1186/s12967-018-1480-0
Basile DP, Anderson MD, Sutton TA (2012) Pathophysiology of acute kidney injury. Compr Physiol 2:1303–1353. https://doi.org/10.1002/cphy.c110041
Evans RG, Ince C, Joles JA, Smith DW, May CN, O'Connor PM, Gardiner BS (2013) Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin Exp Pharmacol Physiol 40:106–122. https://doi.org/10.1111/1440-1681.12031
Audard V, Moutereau S, Vandemelebrouck G, Habibi A, Khellaf M, Grimbert P, Levy Y, Loric S, Renaud B, Lang P, Godeau B, Galacteros F, Bartolucci P (2014) First evidence of subclinical renal tubular injury during sickle-cell crisis. Orphanet J Rare Dis 9:67. https://doi.org/10.1186/1750-1172-9-67
Timsit MO, Tullius SG (2011) Hypothermic kidney preservation: a remembrance of the past in the future? Curr Opin Organ Transplant 16:162–168. https://doi.org/10.1097/MOT.0b013e3283446b07
Kusaka J, Koga H, Hagiwara S, Hasegawa A, Kudo K, Noguchi T (2012) Age-dependent responses to renal ischemia-reperfusion injury. J Surg Res 172:153–158. https://doi.org/10.1016/j.jss.2010.08.034
Miya M, Maeshima A, Mishima K, Sakurai N, Ikeuchi H, Kuroiwa T, Hiromura K, Nojima Y (2012) Age-related decline in label-retaining tubular cells: implication for reduced regenerative capacity after injury in the aging kidney. Am J Physiol Renal Physiol 302:F694–F702. https://doi.org/10.1152/ajprenal.00249.2011
Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, Shi S, Li J, Xie Y, Lu Y, Wang Z (2005) Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci 60:830–839. https://doi.org/10.1093/gerona/60.7.830
Clements ME, Chaber CJ, Ledbetter SR, Zuk A (2013) Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 8:e70464. https://doi.org/10.1371/journal.pone.0070464
Dyess DL, Powell RW, Roberts WS, Tacchi EJ, Swafford AN Jr, Ferrara JJ, Ardell JL (1995) Regional blood flow redistribution in preterm piglets with hemorrhage and resuscitation. J Surg Res 59:29–34. https://doi.org/10.1006/jsre.1995.1128
Maringer K, Sims-Lucas S (2016) The multifaceted role of the renal microvasculature during acute kidney injury. Pediatr Nephrol 31:1231–1240. https://doi.org/10.1007/s00467-015-3231-2
Pham PT, Pham PC, Wilkinson AH, Lew SQ (2000) Renal abnormalities in sickle cell disease. Kidney Int 57:1–8. https://doi.org/10.1046/j.1523-1755.2000.00806.x
Nath KA, Grande JP, Croatt AJ, Frank E, Caplice NM, Hebbel RP, Katusic ZS (2005) Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am J Pathol 166:963–972. https://doi.org/10.1016/S0002-9440(10)62318-8
Wong WS, Moss AA, Federle MP, Cochran ST, London SS (1984) Renal infarction: CT diagnosis and correlation between CT findings and etiologies. Radiology 150:201–205. https://doi.org/10.1148/radiology.150.1.6689761
Deux JF, Audard V, Brugieres P, Habibi A, Manea EM, Guillaud-Danis C, Godeau B, Galacteros F, Stehle T, Lang P, Grimbert P, Audureau E, Rahmouni A, Bartolucci P (2017) Magnetic resonance imaging assessment of kidney oxygenation and perfusion during sickle cell vaso-occlusive crises. Am J Kidney Dis 69:51–59. https://doi.org/10.1053/j.ajkd.2016.07.027
Alaro D, Bashir A, Musoke R, Wanaiana L (2014) Prevalence and outcomes of acute kidney injury in term neonates with perinatal asphyxia. Afr Health Sci 14:682–688. https://doi.org/10.4314/ahs.v14i3.26
Humes HD, Liu S (1994) Cellular and molecular basis of renal repair in acute renal failure. J Lab Clin Med 124:749–754
Csaicsich D, Russo-Schlaff N, Messerschmidt A, Weninger M, Pollak A, Aufricht C (2008) Renal failure, comorbidity and mortality in preterm infants. Wien Klin Wochenschr 120:153–157. https://doi.org/10.1007/s00508-008-0941-5
Yuan SM (2018) Acute kidney injury after pediatric cardiac surgery. Pediatr Neonatol. https://doi.org/10.1016/j.pedneo.2018.03.007
Joffe R, Al Aklabi M, Bhattacharya S, Cave D, Calleja T, Garros D, Majesic N, Ryerson L, Morgan C (2018) Cardiac surgery-associated kidney injury in children and renal oximetry. Pediatr Crit Care Med 19:839–845. https://doi.org/10.1097/PCC.0000000000001656
Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, Wainright JL, Haynes CR, Snyder JJ, Kasiske BL, Israni AK (2018) OPTN/SRTR 2016 annual data report: kidney. Am J Transplant 18:18–113. https://doi.org/10.1111/ajt.14557
Dale-Shall AW, Smith JM, McBride MA, Hingorani SR, McDonald RA (2009) The relationship of donor source and age on short- and long-term allograft survival in pediatric renal transplantation. Pediatr Transplant 13:711–718. https://doi.org/10.1111/j.1399-3046.2008.01054.x
Kusaka M, Kubota Y, Sasaki H, Fukami N, Fujita T, Hirose Y, Takahashi H, Kenmochi T, Shiroki R, Hoshinaga K (2016) Combined predictive value of the expanded donor criteria for long-term graft survival of kidneys from donors after cardiac death: a single-center experience over three decades. Int J Urol 23:319–324. https://doi.org/10.1111/iju.13045
Basile DP (2007) The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72:151–156. https://doi.org/10.1038/sj.ki.5002312
Bornstein P (1995) Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 130:503–506. https://doi.org/10.1083/jcb.130.3.503
Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD (2006) CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 281:26069–26080. https://doi.org/10.1074/jbc.M605040200
Gonzalez-Domenech CM, Munoz-Chapuli R (2010) Molecular evolution of nitric oxide synthases in metazoans. Comp Biochem Physiol Part D Genomics Proteomics 5:295–301. https://doi.org/10.1016/j.cbd.2010.08.004
Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS (2010) Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 88:471–481. https://doi.org/10.1093/cvr/cvq218
Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD (2005) Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A 102:13141–13146. https://doi.org/10.1073/pnas.0502977102
Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD (2008) Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 111:613–623. https://doi.org/10.1182/blood-2007-06-098392
Rogers NM, Seeger F, Garcin ED, Roberts DD, Isenberg JS (2014) Regulation of soluble guanylate cyclase by matricellular thrombospondins: implications for blood flow. Front Physiol 5:134. https://doi.org/10.3389/fphys.2014.00134
Ramanathan S, Mazzalupo S, Boitano S, Montfort WR (2011) Thrombospondin-1 and angiotensin II inhibit soluble guanylyl cyclase through an increase in intracellular calcium concentration. Biochemistry 50:7787–7799. https://doi.org/10.1021/bi201060c
Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD (2010) Thrombospondin-1 inhibits vascular endothelial growth factor receptor-2 signaling by disrupting its association with CD47. J Biol Chem 285:38923–38932. https://doi.org/10.1074/jbc.M110.172304
Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ (2012) Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol 32:2966–2973. https://doi.org/10.1161/ATVBAHA.112.300031
Yao M, Rogers NM, Csanyi G, Rodriguez AI, Ross MA, St Croix C, Knupp H, Novelli EM, Thomson AW, Pagano PJ, Isenberg JS (2014) Thrombospondin-1 activation of signal-regulatory protein-alpha stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol 25:1171–1186. https://doi.org/10.1681/ASN.2013040433
Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD (2008) Thrombospondin-1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity by differentiated U937 cells. Cancer Res 68:7090–7099. https://doi.org/10.1158/0008-5472.CAN-08-0643
Miller YE, Daniels GL, Jones C, Palmer DK (1987) Identification of a cell-surface antigen produced by a gene on human chromosome 3 (cen-q22) and not expressed by Rhnull cells. Am J Hum Genet 41:1061–1070
Mawby WJ, Holmes CH, Anstee DJ, Spring FA, Tanner MJ (1994) Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J 304:525–530. https://doi.org/10.1042/bj3040525
Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N (2009) Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci U S A 106:17413–17418. https://doi.org/10.1073/pnas.0909296106
Ohnishi H, Kaneko Y, Okazawa H, Miyashita M, Sato R, Hayashi A, Tada K, Nagata S, Takahashi M, Matozaki T (2005) Differential localization of Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 and CD47 and its molecular mechanisms in cultured hippocampal neurons. J Neurosci 25:2702–2711. https://doi.org/10.1523/JNEUROSCI.5173-04.2005
Soto-Pantoja DR, Kaur S, Roberts DD (2015) CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol 50:212–230. https://doi.org/10.3109/10409238.2015.1014024
Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, Willingham S, Howard M, Prohaska S, Volkmer J, Chao M, Weissman IL, Majeti R (2015) Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One 10:e0137345. https://doi.org/10.1371/journal.pone.0137345
Murata Y, Saito Y, Kotani T, Matozaki T (2018) CD47-signal regulatory protein alpha signaling system and its application to cancer immunotherapy. Cancer Sci 109:2349–2357. https://doi.org/10.1111/cas.13663
Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P (1993) Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn 197:40–56. https://doi.org/10.1002/aja.1001970105
Wahab NA, Schaefer L, Weston BS, Yiannikouris O, Wright A, Babelova A, Schaefer R, Mason RM (2005) Glomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia 48:2650–2660. https://doi.org/10.1007/s00125-005-0006-5
Yoo KH, Yim HE, Bae ES, Hong YS (2018) Capillary rarefaction and altered renal development: the imbalance between pro- and anti-angiogenic factors in response to angiotensin II inhibition in the developing rat kidney. J Mol Histol 49:219–228. https://doi.org/10.1007/s10735-018-9762-7
Hugo C, Shankland SJ, Pichler RH, Couser WG, Johnson RJ (1998) Thrombospondin 1 precedes and predicts the development of tubulointerstitial fibrosis in glomerular disease in the rat. Kidney Int 53:302–311. https://doi.org/10.1046/j.1523-1755.1998.00774.x
Hugo C, Daniel C (2009) Thrombospondin in renal disease. Nephron Exp Nephrol 111:e61–e66. https://doi.org/10.1159/000198235
Kosugi T, Heinig M, Nakayama T, Connor T, Yuzawa Y, Li Q, Hauswirth WW, Grant MB, Croker BP, Campbell-Thompson M, Zhang L, Atkinson MA, Segal MS, Nakagawa T (2009) Lowering blood pressure blocks mesangiolysis and mesangial nodules, but not tubulointerstitial injury, in diabetic eNOS knockout mice. Am J Pathol 174:1221–1229. https://doi.org/10.2353/ajpath.2009.080605
Daniel C, Schaub K, Amann K, Lawler J, Hugo C (2007) Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes 56:2982–2989. https://doi.org/10.2337/db07-0551
Hafdi Z, Lesavre P, Nejjari M, Halbwachs-Mecarelli L, Droz D, Noel LH (2000) Distribution of alphavbeta3, alphavbeta5 integrins and the integrin associated protein--IAP (CD47) in human glomerular diseases. Cell Adhes Commun 7:441–451
Rogers NM, Thomson AW, Isenberg JS (2012) Activation of parenchymal CD47 promotes renal ischemia-reperfusion injury. J Am Soc Nephrol 23:1538–1550. https://doi.org/10.1681/ASN.2012020137
Bijuklic K, Sturn DH, Jennings P, Kountchev J, Pfaller W, Wiedermann CJ, Patsch JR, Joannidis M (2006) Mechanisms of neutrophil transmigration across renal proximal tubular HK-2 cells. Cell Physiol Biochem 17:233–244. https://doi.org/10.1159/000094128
Kurihara H, Harita Y, Ichimura K, Hattori S, Sakai T (2010) SIRP-alpha-CD47 system functions as an intercellular signal in the renal glomerulus. Am J Physiol Renal Physiol 299:F517–F527. https://doi.org/10.1152/ajprenal.00571.2009
Shinohara M, Ohyama N, Murata Y, Okazawa H, Ohnishi H, Ishikawa O, Matozaki T (2006) CD47 regulation of epithelial cell spreading and migration, and its signal transduction. Cancer Sci 97:889–895. https://doi.org/10.1111/j.1349-7006.2006.00245.x
Nishiyama Y, Tanaka T, Naitoh H, Mori C, Fukumoto M, Hiai H, Toyokuni S (1997) Overexpression of integrin-associated protein (CD47) in rat kidney treated with a renal carcinogen, ferric nitrilotriacetate. Jpn J Cancer Res 88:120–128. https://doi.org/10.1111/j.1349-7006.1997.tb00356.x
Thompson EM, Hughes J, Van Noorden S, Sharpe J, Savill J (1996) Expression of the multifunctional extracellular matrix protein thrombospondin in crescentic glomerulonephritis. J Pathol 178:89–94. https://doi.org/10.1002/(SICI)1096-9896(199601)178:1<89::AID-PATH457>3.0.CO;2-4
Suzuma K, Takagi H, Otani A, Oh H, Honda Y (1999) Expression of thrombospondin-1 in ischemia-induced retinal neovascularization. Am J Pathol 154:343–354. https://doi.org/10.1016/S0002-9440(10)65281-9
Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N (2003) Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn 228:630–642. https://doi.org/10.1002/dvdy.10412
Shafiee A, Penn JS, Krutzsch HC, Inman JK, Roberts DD, Blake DA (2000) Inhibition of retinal angiogenesis by peptides derived from thrombospondin-1. Invest Ophthalmol Vis Sci 41:2378–2388
Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY (2003) Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke 34:177–186. https://doi.org/10.1161/01.STR.0000047100.84604.BA
Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML (2005) Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 111:2935–2942. https://doi.org/10.1161/CIRCULATIONAHA.104.510354
Sezaki S, Hirohata S, Iwabu A, Nakamura K, Toeda K, Miyoshi T, Yamawaki H, Demircan K, Kusachi S, Shiratori Y, Ninomiya Y (2005) Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp Biol Med (Maywood) 230:621–630
Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD (2008) Treatment of ischemia/reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery 144:752–761. https://doi.org/10.1016/j.surg.2008.07.009
Causey MW, Salgar S, Singh N, Martin M, Stallings JD (2012) Valproic acid reversed pathologic endothelial cell gene expression profile associated with ischemia-reperfusion injury in a swine hemorrhagic shock model. J Vasc Surg 55:1096–1103 e1051. https://doi.org/10.1016/j.jvs.2011.08.060
Freyberg MA, Kaiser D, Graf R, Buttenbender J, Friedl P (2001) Proatherogenic flow conditions initiate endothelial apoptosis via thrombospondin-1 and the integrin-associated protein. Biochem Biophys Res Commun 286:141–149. https://doi.org/10.1006/bbrc.2001.5314
Ennis WJ, Koh TJ, Urao N, Jan YK, Sui A, Brown K, Borhani M (2015) Ischemia/reperfusion: a potential cause for tissue necrosis. In: Téot L, Meaume S, Akita S, Ennis WJ, del Marmol V (eds) Skin Necrosis. Springer, Vienna, pp. 9–17. https://doi.org/10.1007/978-3-7091-1241-0_2
Isenberg JS, Shiva S, Gladwin M (2009) Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide 21:52–62. https://doi.org/10.1016/j.niox.2009.05.005
Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD (2009) Thrombospondin-1-CD47 blockade following ischemia reperfusion injury is tissue protective. Plast Reconstr Surg 124:1880–1889. https://doi.org/10.1097/PRS.0b013e3181bceec3
Wang HB, Yang J, Ding JW, Chen LH, Li S, Liu XW, Yang CJ, Fan ZX, Yang J (2016) RNAi-mediated down-regulation of CD47 protects against ischemia/reperfusion-induced myocardial damage via activation of eNOS in a rat model. Cell Physiol Biochem 40:1163–1174. https://doi.org/10.1159/000453170
Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, Roberts DD (2008) Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: implications for human disease. Ann Surg 247:860–868. https://doi.org/10.1097/SLA.0b013e31816c4006
Xiao ZY, Banan B, Jia J, Manning PT, Hiebsch RR, Gunasekaran M, Upadhya GA, Frazier WA, Mohanakumar T, Lin Y, Chapman WC (2015) CD47 blockade reduces ischemia/reperfusion injury and improves survival in a rat liver transplantation model. Liver Transpl 21:468–477. https://doi.org/10.1002/lt.24059
Talukder MA, Yang F, Shimokawa H, Zweier JL (2010) eNOS is required for acute in vivo ischemic preconditioning of the heart: effects of ischemic duration and sex. Am J Physiol Heart Circ Physiol 299:H437–H445. https://doi.org/10.1152/ajpheart.00384.2010
Kerkela R, Karsikas S, Szabo Z, Serpi R, Magga J, Gao E, Alitalo K, Anisimov A, Sormunen R, Pietila I, Vainio L, Koch WJ, Kivirikko KI, Myllyharju J, Koivunen P (2013) Activation of hypoxia response in endothelial cells contributes to ischemic cardioprotection. Mol Cell Biol 33:3321–3329. https://doi.org/10.1128/MCB.00432-13
Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD (2009) Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol 28:110–119. https://doi.org/10.1016/j.matbio.2009.01.002
Narizhneva NV, Razorenova OV, Podrez EA, Chen J, Chandrasekharan UM, DiCorleto PE, Plow EF, Topol EJ, Byzova TV (2005) Thrombospondin-1 up-regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J 19:1158–1160. https://doi.org/10.1096/fj.04-3310fje
Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, Frazier WA, Roberts DD (2007) Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol 27:2582–2588. https://doi.org/10.1161/ATVBAHA.107.155390
Suzuki K, Wang R, Kubota H, Shibuya H, Saegusa J, Sato T (2005) Kinetics of biglycan, decorin and thrombospondin-1 in mercuric chloride-induced renal tubulointerstitial fibrosis. Exp Mol Pathol 79:68–73. https://doi.org/10.1016/j.yexmp.2005.01.010
Maimaitiyiming H, Zhou Q, Wang S (2016) Thrombospondin 1 deficiency ameliorates the development of adriamycin-induced proteinuric kidney disease. PLoS One 11:e0156144. https://doi.org/10.1371/journal.pone.0156144
Xie XS, Li FY, Liu HC, Deng Y, Li Z, Fan JM (2010) LSKL, a peptide antagonist of thrombospondin-1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch Pharm Res 33:275–284. https://doi.org/10.1007/s12272-010-0213-6
Sun D, Ma Y, Han H, Yin Z, Liu C, Feng J, Zhou X, Li X, Xiao A, Yu R (2012) Thrombospondin-1 short hairpin RNA suppresses tubulointerstitial fibrosis in the kidney of ureteral obstruction by ameliorating peritubular capillary injury. Kidney Blood Press Res 35:35–47. https://doi.org/10.1159/000330718
Wang J, Du Z, Zhang W, Han B, Peng C, Chen N (2011) Post liver transplantation acute kidney injury in a rat model of syngeneic orthotopic liver transplantation. Lab Investig 91:1158–1169. https://doi.org/10.1038/labinvest.2011.59
Jung SH, Hwang JH, Kim SE, Young Kyu K, Park HC, Lee HT (2017) The potentiating effect of hTFPI in the presence of hCD47 reduces the cytotoxicity of human macrophages. Xenotransplantation 24. https://doi.org/10.1111/xen.12301
Dai H, Friday AJ, Abou-Daya KI, Williams AL, Mortin-Toth S, Nicotra ML, Rothstein DM, Shlomchik WD, Matozaki T, Isenberg JS, Oberbarnscheidt MH, Danska JS, Lakkis FG (2017) Donor SIRPalpha polymorphism modulates the innate immune response to allogeneic grafts. Sci Immunol 2. DOI: https://doi.org/10.1126/sciimmunol.aam6202
Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M (2005) Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 115:3451–3459. https://doi.org/10.1172/JCI25461
Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD (2007) Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem 282:15404–15415. https://doi.org/10.1074/jbc.M701638200
Zager RA, Johnson AC, Hanson SY, Shah VO (2003) Acute tubular injury causes dysregulation of cellular cholesterol transport proteins. Am J Pathol 163:313–320. https://doi.org/10.1016/S0002-9440(10)63655-3
Isenberg JS, Yu C, Roberts DD (2008) Differential effects of ABT-510 and a CD36-binding peptide derived from the type 1 repeats of thrombospondin-1 on fatty acid uptake, nitric oxide signaling, and caspase activation in vascular cells. Biochem Pharmacol 75:875–882. https://doi.org/10.1016/j.bcp.2007.10.025
Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD (2007) Increasing survival of ischemic tissue by targeting CD47. Circ Res 100:712–720. https://doi.org/10.1161/01.RES.0000259579.35787.4e
Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD (2008) Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg 247:180–190. https://doi.org/10.1097/SLA.0b013e31815685dc
Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD (2009) Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 284:1116–1125. https://doi.org/10.1074/jbc.M804860200
Wang J, Long Q, Zhang W, Chen N (2012) Protective effects of exogenous interleukin 18-binding protein in a rat model of acute renal ischemia-reperfusion injury. Shock 37:333–340. https://doi.org/10.1097/SHK.0b013e318240bdc8
Kim J, Kim C, Kim TS, Bang SI, Yang Y, Park H, Cho D (2006) IL-18 enhances thrombospondin-1 production in human gastric cancer via JNK pathway. Biochem Biophys Res Commun 344:1284–1289. https://doi.org/10.1016/j.bbrc.2006.04.016
Rogers NM, Yao M, Novelli EM, Thomson AW, Roberts DD, Isenberg JS (2012) Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. Am J Physiol Renal Physiol 303:F1117–F1125. https://doi.org/10.1152/ajprenal.00359.2012
Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Isenberg JS, Roberts DD (2013) Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 3:1673. https://doi.org/10.1038/srep01673
Rogers NM, Zhang ZJ, Wang JJ, Thomson AW, Isenberg JS (2016) CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Int 90:334–347. https://doi.org/10.1016/j.kint.2016.03.034
van Beek EM, Zarate JA, van Bruggen R, Schornagel K, Tool AT, Matozaki T, Kraal G, Roos D, van den Berg TK (2012) SIRPalpha controls the activity of the phagocyte NADPH oxidase by restricting the expression of gp91(phox). Cell Rep 2:748–755. https://doi.org/10.1016/j.celrep.2012.08.027
Meijles DN, Sahoo S, Al Ghouleh I, Amaral JH, Bienes-Martinez R, Knupp HE, Attaran S, Sembrat JC, Nouraie SM, Rojas MM, Novelli EM, Gladwin MT, Isenberg JS, Cifuentes-Pagano E, Pagano PJ (2017) The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci Signal 10. https://doi.org/10.1126/scisignal.aaj1784
Lario S, Bescos M, Campos B, Mur C, Luque P, Alvarez R, Campistol JM (2007) Thrombospondin-1 mRNA expression in experimental kidney transplantation with heart-beating and non-heart-beating donors. J Nephrol 20:588–595
Delpech PO, Thuillier R, Le Pape S, Rossard L, Jayle C, Billault C, Goujon JM, Hauet T (2014) Effects of warm ischaemia combined with cold preservation on the hypoxia-inducible factor 1alpha pathway in an experimental renal autotransplantation model. Br J Surg 101:1739–1750. https://doi.org/10.1002/bjs.9611
Mas VR, Mas LA, Archer KJ, Yanek K, King AL, Gibney EM, Cotterell A, Fisher RA, Posner M, Maluf DG (2007) Evaluation of gene panel mRNAs in urine samples of kidney transplant recipients as a non-invasive tool of graft function. Mol Med 13:315–324. https://doi.org/10.2119/2007-00017.Mas
Hotchkiss H, Chu TT, Hancock WW, Schroppel B, Kretzler M, Schmid H, Liu Y, Dikman S, Akalin E (2006) Differential expression of profibrotic and growth factors in chronic allograft nephropathy. Transplantation 81:342–349. https://doi.org/10.1097/01.tp.0000195773.24217.95
Lin Y, Manning PT, Jia J, Gaut JP, Xiao Z, Capoccia BJ, Chen CC, Hiebsch RR, Upadhya G, Mohanakumar T, Frazier WA, Chapman WC (2014) CD47 blockade reduces ischemia-reperfusion injury and improves outcomes in a rat kidney transplant model. Transplantation 98:394–401. https://doi.org/10.1097/TP.0000000000000252
Xu M, Wang X, Banan B, Chirumbole DL, Garcia-Aroz S, Balakrishnan A, Nayak DK, Zhang Z, Jia J, Upadhya GA, Gaut JP, Hiebsch R, Manning PT, Wu N, Lin Y, Chapman WC (2018) Anti-CD47 monoclonal antibody therapy reduces ischemia-reperfusion injury of renal allografts in a porcine model of donation after cardiac death. Am J Transplant 18:855–867. https://doi.org/10.1111/ajt.14567
Hugo C, Kang DH, Johnson RJ (2002) Sustained expression of thrombospondin-1 is associated with the development of glomerular and tubulointerstitial fibrosis in the remnant kidney model. Nephron 90:460–470. https://doi.org/10.1159/000054735
Hohenstein B, Daniel C, Wittmann S, Hugo C (2008) PDE-5 inhibition impedes TSP-1 expression, TGF-beta activation and matrix accumulation in experimental glomerulonephritis. Nephrol Dial Transplant 23:3427–3436. https://doi.org/10.1093/ndt/gfn319
Aibara N, Ohyama K, Hidaka M, Kishikawa N, Miyata Y, Takatsuki M, Eguchi S, Kuroda N (2018) Immune complexome analysis of antigens in circulating immune complexes from patients with acute cellular rejection after living donor liver transplantation. Transpl Immunol 48:60–64. https://doi.org/10.1016/j.trim.2018.02.011
Gholamin S, Mitra SS, Feroze AH, Liu J, Kahn SA, Zhang M, Esparza R, Richard C, Ramaswamy V, Remke M, Volkmer AK, Willingham S, Ponnuswami A, McCarty A, Lovelace P, Storm TA, Schubert S, Hutter G, Narayanan C, Chu P, Raabe EH, Harsh Gt, Taylor MD, Monje M, Cho YJ, Majeti R, Volkmer JP, Fisher PG, Grant G, Steinberg GK, Vogel H, Edwards M, Weissman IL, Cheshier SH (2017) Disrupting the CD47-SIRPalpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 9. DOI: https://doi.org/10.1126/scitranslmed.aaf2968
Funding
This study was funded in part by the Intramural Research Program of the NIH, NCI, CCR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D.D.R. declares no conflict of interest. J.S.I. serves as a consultant to Radiation Control Technologies, Inc.
Rights and permissions
About this article
Cite this article
Isenberg, J.S., Roberts, D.D. The role of CD47 in pathogenesis and treatment of renal ischemia reperfusion injury. Pediatr Nephrol 34, 2479–2494 (2019). https://doi.org/10.1007/s00467-018-4123-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4123-z