Abstract
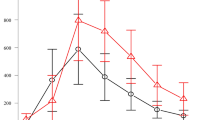
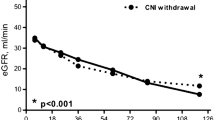
Clinical trials in adult liver and heart recipients have shown that management of cyclosporine (CsA) dose with 2-h levels (C2) leads to lower rejection rates and serum creatinine levels compared with C0 monitoring. Therefore, we investigated whether C2 monitoring might also improve late graft survival after kidney transplantation in children. To date, no results in adult renal transplantation and in pediatric transplantation have been published. Forty-nine stable pediatric kidney recipients with a minimum time of 1 year after transplantation (mean=7±5 years) entered the study. None of the patients had experienced an acute rejection up to 6 months before entering the study. CsA dosing was based on C0 monitoring for the first 6 months and then based on C2 monitoring for the following 6 months. C0 and C2 levels were measured at 4-weekly intervals. Percentage decline in glomerular filtration rate (GFR) and mean coefficients of variation of CsA levels (Cvar) were calculated and compared during the 6-months periods. At the beginning of the study, the mean calculated GFR was 53±15 ml/min per 1.73 m2. During the 6 months of C0 monitoring, the mean GFR decreased to 49±12 m/min per 1.73 m2 (P=0.001, paired t-test). Six months after switching to C2 monitoring, the mean GFR remained stable, at 49±15 ml/min per 1.73 m2 (P=0.3 paired t-test). The largest increase in GFR (3.9±7.9%) was found in patients with a decrease of their CsA dose of more than 5% under C2 monitoring. Cvar was significantly lower under C2 than under C0 monitoring (0.24±0.10 vs. 0.30±0.15, P=0.02, unpaired t-test). We conclude that the switch to C2 monitoring helped to identify patients with CsA overdosing as well as to reduce variation in CsA level, which resulted in a halt in GFR decline.

Similar content being viewed by others
References
Levy G, Burra P, Cavallari A, Duvoux C, Lake J, Mayer AD, Mies S, Pollard SG, Varo E, Villamil F, Johnston A (2002). Improved clinical outcome for liver transplant recipients using cylosporine monitoring based on 2-hr post-dose levels (C2). Transplantation 73:953–959
Cantarovich M, Elstein E, Varennes B de, Barkun JS (1999) Clinical benefit of neoral dose monitoring with cyclosporine-2 hr-post-dose levels compared with trough levels in stable heart transplant patients. Transplantation 68:1839–1842
Cantarovic M, Barkun JS, Tchervenkov JI, Besner JG, Aspeslet L, Metrakos P (1998) Comparison of neoral dose monitoring with cyclosporine rough levels versus 2-hr post-dose levels in stable liver transplant patients. Transplantation 66:1621–1627
Keshavamurthy M, Al Ahmadi I, Mohammed Raza S, Baynton R, Ali Meshari K, Al Shabani K (2001) Single-center study utilizing C(2) level as monitoring tool in de novo renal transplant recipients treated with Neoral. Transplant Proc 33:3112–3114
Maham N, Cardella C, Cole E, Cattran D, Fenton S, O’Grady C, Hamil S, Smith R, Cole E (2001) Optimization of cyclosporine exposure utilizing C(2)-level monitoring in de novo renal transplant recipients. The Toronto General Hospital experience. Transplant Proc 33:3098–3099
Canadian Neoral Renal Transplantation Study Group (2001) Absorption profile of cyclosporine microemulsion (Neoral) during the first two weeks after renal transplantation. Transplantation 72:1024–1032
Nashan B, Cole E, Levy G, Thervet E (2002) Clinical validation studies of Neoral C(2)-monitoring. A review. Transplantation 73:S3–S11
Mahalati K, Belitsky P, West K, Kiberd B, Fraser A, Shetris I, Macdonald AS, McAlister V, Lawen J (2001) Approaching the therapeutic window for cyclosporine in kidney transplantation: a prospective study. J Am Soc Nephrol 12:823–833
Cole E, Midtvedt K, Johnston A, Pattison J, O’Grady C (2002) Recommendations for the implementation of Neoral C(2)-monitoring in clinical practice. Transplantation 73:S19–S22
Levy G, Thervet E, Lake J, Uchida K (2002) Consensus on Neoral (c2): Expert Review in Transplantation (CONCERT) Group. Patient management by Neoral C2 monitoring: an international consensus statement. Transplantation 73:S12–S18
International Neoral Transplantation Study Group (2002) Randomized, international study of cyclosporine microemulsion absorption profiling in renal transplantation with basiliximab immunoprophylaxis. Am J Transplant 2:157–166
Meier-Kriesche HU, Swinford R, Kahan BD, Brannan P, Portman RJ (2000) Reduced variability of neoral pharmacokinetic studies in pediatric renal transplantation. Peditatr Nephrol 15:2–6
Dello Strologo L, Campagnano P, Federici G, Rizzoni G (1999) Cyclosporine A monitoring in children: abbreviated area under curve formulas and C2 level. Pediatr Nephrol 13:95–97
International Neoral Transplantation Study Group (2002) Cyclosporine microemulsion (Neoral) absorption profiling and sparse-sample predictors during the first 3 months after renal transplantation. Am J Transplant 2:148–156
Filler G, Mai I, Filler S, Ehrich JHH (1999) Abbreviated cyclosporine AUCs on Neoral—the search continues. Pediatr Nephrol 13:98–102
Hoyer PF, Vester U (2001) Refining immunosuppressive protocols in pediatric renal transplant recipients. Transplant Proc 33:3587–3589
Kelles A, Herman J, Tjandra-Maga TB, Van Damme-Lombaerts R (1999) Sandimmune to Neoral conversion and value of abbreviated AUC monitoring in stable pediatric kidney transplant recipients. Pediatr Transplant 3:282–287
Wigger M, Druckler E, Muscheites J, Stolpe HJ, Kundt G, Wacke R (2001) Cyclosporine peak concentration in relation to the four-hour absorption phase in pediatric renal graft recipients. Transplant Proc 33:3126–3127
Dunn S, Falkenstein K, Cooney G (2001) Neoral C2 monitoring in pediatric liver transplant recipients. Transplant Proc 33:3094–3095
Meier-Kriesche HU, Bonilla-Felix MA, Ferris ME, Swinford R, Kahan BD, Brannan P, Portman RJ (1999) A limited sampling strategy for the estimation of Neoral AUCs in pediatric patients. Pediatr Nephrol 13:742–747
Hoyer PF (2000) Therapeutic drug monitoring of cyclosporine A: should we use the area under the concentration time-curve and forget trough levels? Pediatr Transplant 4:2–5
Dunn SP (2003) Neoral monitoring 2 hours post-dose and the pediatric transplant recipient. Pediatr Transplant 7:25–30
Pape L, Lehnhardt A, Latta K, Ehrich JHH, Offner G (2003) Cyclosporin A monitoring by two hour levels: preliminary target levels in stable pediatric kidney transplant recipients. Clin Transpl 17:546-548
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571–590
Cole E, Maham N, Cardella C, Cattran D, Fenton S, Hamel J, O’Grady C, Smith R (2003) Clinical benefits of neoral C2 monitoring in the long-term management of renal transplant recipients. Transplantation 27:2086–2090
Bokenkamp A, Offner G, Hoyer PF, Wonigeit K, Brodehl J (1996) Excessive cyclosporine trough level variation with classic cyclosporine can be reduced with the new oral microemulsion formulation of cyclosporine. Transplant Proc 28:2273–2275
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pape, L., Ehrich, J.H.H. & Offner, G. Advantages of cyclosporin A using 2-h levels in pediatric kidney transplantation. Pediatr Nephrol 19, 1035–1038 (2004). https://doi.org/10.1007/s00467-004-1481-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1481-5