Abstract
Background
Open pancreatoduodenectomy with vein resection (OPD-VR) is now standard of care in patients who responded to neoadjuvant therapies. Feasibility of robotic pancreatoduodenectomy (RPD) with vein resection (RPD-VR) was shown, but no study provided a detailed description of the technical challenges associated with this formidable operation. Herein, we describe the trips and tricks for technically successful RPD-VR.
Methods
The vascular techniques used in RPD-VR were borrowed from OPD-VR, as well as from our experience with robotic transplantation of both kidney and pancreas. Vein resection was classified into 4 types according to the international study group of pancreatic surgery. Each type of vein resection was described in detail and shown in a video.
Results
Between October 2008 and November 2021, a total of 783 pancreatoduodenectomies were performed, including 233 OPDs-VR (29.7%). RPD was performed in 256 patients (32.6%), and RPDs-VR in 36 patients (4.5% of all pancreatoduodenectomies; 15.4% of all pancreatoduodenectomies with vein resection; 14.0% of all RPDs). In RPD-VR vein resections were: 4 type 1 (11.1%), 10 type 2 (27.8%), 12 type 3 (33.3%) and 10 type 4 (27.8%). Vascular patches used in type 2 resections were made of peritoneum (n = 8), greater saphenous vein (n = 1), and deceased donor aorta (n = 1). Interposition grafts used in type 4 resections were internal left jugular vein (n = 8), venous graft from deceased donor (n = 1) and spiral saphenous vein graft (n = 1).
There was one conversion to open surgery (2.8%). Ninety-day mortality was 8.3%. There was one (2.8%) partial vein thrombosis, treated with heparin infusion.
Conclusions
We have reported 36 technically successful RPDs-VR. We hope that the tips and tricks provided herein can contribute to safer implementation of RPD-VR. Based on our experience, and according to data from the literature, we strongly advise that RPD-VR is performed by expert surgeons at high volume centers.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
After a long debate, and thanks to the availability of effective chemotherapy regimens, vein resection in pancreatectomy for pancreatic cancer is now considered standard of care [1, 2]. These operations are typically performed through an open approach that permits straightforward vascular reconstruction. Type of vein resection and reconstruction is classified according to the International Study Group of Pancreatic Surgery (ISGPS) [2]. In the open setting many vascular resections involve a vein segment and recostruction is frequently achieved by end-to-end anastomosis [3].
Recently, some groups have reported vein resection and reconstruction in minimally invasive pancreatoduodenectomy (MIPD) [4, 9]. However, even when using robotic assistance, which is known to facilitate intracorporeal sutures [10], most MIPDs with vein resection consist in small side-bite vascular resections that are often managed by an endoscopic stapler [4]. In MIPD other types of vein resection and reconstruction are feasible, but at the price of increased operative difficulty [5,6,7,8,9].
Involvement of the spleno-mesenteric junction is probably the most difficult scenario to manage in MIPD. Even when key collateral circulation is spared, ligature of the splenic vein results in severe sinistral portal hypertension in approximately one third of the patients. In open pancreatoduodenectomy (OPD), the splenic vein can be quite easily anastomosed to the left renal vein [3], while direct reconstruction of the superior mesenteric/portal vein (SMV/PV) is facilitated by either liver or intestinal mobilization [11]. All these maneuvers are difficult to implement in MIPD.
Our group has pioneered vascular resections in pancreatic cancer [12,13,14], having now performed over 700 open pancreatectomies with resection ± reconstruction. We implemented laparoscopic distal pancreatectomy in the late’90 s [15] and performed the first robotic pancreatoduodenectomy (RPD) in 2008 [16]. Thereafter, we have reported the feasibility of either vein [6] and artery [7] resections during RPD. Now, after some more experience, we wish to report on tips and tricks for RPD with vein resection (RPD-VR).
Methods
Selection criteria
Our selection criteria for RPD were previously reported [16, 17]. In general, as more experience was gained, we have gradually expanded our selection criteria. As specifically regards, RPD-VR, we still consider an absolute contraindication vein involvement ≥ 180° as well as segmental vein occlusion and/or vein thrombosis. However, in some patients vein involvement is not suspected until surgical exploration (unplanned vascular resection). In these patients, tumor abutment of the SMV/PV is typically limited. The decision whether to proceed with vein resection or to convert the procedure to open surgery is based on the possibility to proceed safely, while respecting the golden oncological principles established in OPD [12].
Preoperative planning
Preoperative planning, based on careful review of computed tomography scans, is of paramount importance in MIPD. According to the recent expert consensus meeting on precision anatomy for MIPD [18], we carefully check the following items:
-
1.Presence of anatomical variations in arterial liver supply, and branching pattern of hepatic artery(ies);
-
2.Presence of anatomic variations in either superior mesenteric artery (SMA) and superior mesenteric vein (SMV) and their branching patterns;
-
3.Origin and course of inferior pancreaticoduodenal artery, dorsal pancreatic artery, first and second jejunal artery, first jejunal vein, left gastric vein, and inferior mesenteric vein;
-
4.Presence of celiac artery stenosis;
-
5.Presence of circumportal pancreas.
Vascular relationships between tumor and SMV/PV are also carefully noted, as previously described [19]. In addition, in preparation for triangle RPD [20] origin, course and branching pattern of right renal and adrenal arteries are also noted.
Possible sources of vascular grafts
Types of vein resection and reconstruction according to the ISGPS [2] are presented in Fig. 1.
Types of vein resection and reconstruction according to the International Study Group of Pancreatic Surgery. a. Type 1 resection (small side-wall resection with direct repair); b. Type 2 resection (larger side-wall resection with patch repair); c. Type 3 resection (segmental resection with direct repair); d. Type 4 resection (segmental resection; an interposition graft is used for vascular repair)
Before trespassing the point of no return, a strategy for reconstruction must be established. Even when planning for direct repair (type 1 and type 3 resections) the surgeon must have a clear vision of which vascular grafts are available for possible vascular reconstruction. A full set of laparoscopic (or robotic) bulldog vascular clamps must be available, and the surgeon at the table and the scrub nurse must feel confident with their use.
Despite vascular prostheses have been successfully used to repair the SMV/PV [21, 22] we prefer to employ either autologous or allogeneic grafts. In case of type 4 vein resection our first choice is the internal jugular vein [23]. When this vein is not available, the next option is to use a spiral saphenous vein graft [24]. When neither these veins are available, we consider to use a graft from a deceased organ donor [25]. Additional sources of interposition grafts are the left renal vein [26], and the superficial femoral vein [27].
For patch repair (type 2 resection) we still prefer to avoid vascular prostheses. Autologous vascular patches can be obtained from the internal jugular vein, the greater saphenous vein [27], the inferior mesenteric vein [28], the right gonadal vein [29], the parietal peritoneum [30] and the falciform ligament [31]. Again, when these grafts are not available or are not suitable allogeneic grafts from deceased donors can be used. Bovine pericardial grafts can also be used as either tube grafts or vascular patches [4].
Surgical technique
The patient is prepared as for a standard RPD [16, 20]. Part of this standard preparation includes exposure of both groins and the left lateral region of neck, in preparation for procurement of vascular grafts (Fig. 2).
Dissection proceeds following the technique recently reported for the “cold” triangle RPD [20]. The specimen is therefore mobilized from all retroperitoneal attachments, but the area of suspected vein involvement, by proceeding along the adventitial plane of large peripancreatic arteries (Figs. 3 and 4). At this time the surgeon has to decide which type of vein resection and reconstruction to perform.
For running vascular sutures, we prefer to use expanded polytetrafluoroethylene (e-PTFE). To facilitate intracorporeal handling, two suture legs of approximately 12 cm each are tied together to obtain a shorter double armed suture (Fig. 5). Fine sutures are used (6/0 or 7/0).
The SMV/PV is crossclamped using laparoscopic bulldog vascular clamps. The SMA is also crossclamped, to reduce intestinal congestion during vein reconstruction.
After opening the vein, the vessel is flushed with topical sodium heparin injected through an 8 French Bracci ureteral catheter (Coloplast A/S, 3050 Humlebaek, Denmark) connected to a syringe handled by the surgeon at the table. Before completing the anastomosis, the vascular clamp on the SMV is released for a couple of seconds. Afterwards, the vein is flushed again with topical sodium heparin.
When reconstruction time exceeds 30 min, a bolus of unfractionated heparin (60–80 IU/kg body weight) is administered with the aim to increase activated clotting time between 200 and 250 s.
Upon completion of vascular reconstruction, in all types of vein resection, the first vascular clamp to be removed is the one on the portal vein. This maneuver permits to fix bleeding sites at a low pressure. Before removing vascular clamps, we place a small peanut sponge near to the SMV/PV. In case of bleeding the peanut sponge can be used for gentle vascular compression before hemostasis is secured by suture. The peanut sponge is made of oxidized regenerated cellulose. Therefore, it can be reabsorbed. This choice avoids the stressful situation, that might otherwise arise at the end of the procedure, if the (small) sponge was missing.
Type 1 vein resection and reconstruction
Type 1 vein resection is performed when the area of tumor/vein contact is quite limited. In these patients a small side-wall vein resection is performed and vascular repair should not create a stenosis of the SMV-PV. This is why, when using an endoscopic stapler, the device should be fired perpendicular, rather than parallel, to the main axis of the SMV/PV. A multifire endo TA™ stapler (Covidien, Covidien IIc, Mansfiled, MA02048 USA) can be used to spare some vein wall. Alternatively, an endoscopic GIA stapler can also be used (Fig. 6). However, in RPD the short suture required in type 1 resections can be readily performed and the use of a stapler can be avoided (video 1).
Stapled type 1 vein resection. a. Firing the endoscopic stapler parallel to the superior mesenteric/portal vein may create a vein stenosis, possibly leading to turbulence in portal flow; b. Firing the endoscopic stapler perpendicular to the superior mesenteric/portal vein decreases the risk of vein stenosis and therefore of turbulence in portal flow
Type 2 vein resection and reconstruction
A type 2 resection is a larger side-wall resection. Primary closure is not possible and a vascular patch is required. In MIPD, type 2 resection may be particularly indicated when vein resection involves the spleno-mesenteric junction, because it avoids the need to anastomose also the splenic vein. Before proceeding to resection, the size of the patch must be carefully determined. To do so, we match the length of a suture to both longitudinal and transverse diameters of the side-wall vein resection. In RPD handling a large vein patch can complicate workflow. This is why, we prefer to use “more rigid” vascular patches, such as a deceased donor arterial patch. In all patients, a large vein patch is removed en-bloc with the specimen, paying careful attention to keep a safety margin from the area of suspected tumor invasion. A stay suture is placed on the anterior margin of the vascular defect to improve exposure. The posterior wall is sutured from the inside (Fig. 7) (video 2).
Type 2 vein resection. Repair is achieved using a large arterial patch from a deceased donor. a. Excision of a large vein patch en-bloc with the tumor; b. The arterial patch is placed near to the vein breach in preparation for suture; c. The posterior wall is sutured from inside. Note the optimal exposure achieved with the use of the arterial patch; d. Vascular repair completed
Type 3 vein resection and reconstruction
Before proceeding with type 3 vein resection and reconstruction in MIPD, the surgeon must decide if direct reconstruction is possible. However, for safety reasons, a plan for rescue conversion to type 4 reconstruction must be available.
End-to-end vein anastomosis can be done using either two half-running sutures [32] or a parachute technique [33]. At the end, sutures must be tied with a growth factor, as originally described by Starzl. After clamp release migration of the extra suture ensures prompt expansion of the anastomosis thus reducing the risk of stenosis, that otherwise would occur from a purse string effect [32] (Fig. 8). To facilitate direct anastomosis, the degree of reverse Trendelenburg can be slightly decreased and a robotic instrument, holding a small peanut sponge, can be used to push the mesenteric root towards the liver. A type 3 resection is presented in video 3.
Type 4 vein resection and reconstruction
Type 4 vein resection and reconstruction is required when direct vascular reconstruction is not possible.
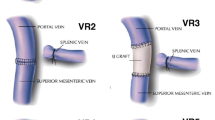
Considering that two anastomoses are required, in type 4 vein resection and reconstruction initially only the SMV is crossclamped to maintain some portal flow through the splenic vein. Actually, if the inferior mesenteric vein drains into the splenic vein, also some venous outflow from the intestine may be maintained (Fig. 9). Once everything is ready for reconstruction the interposition graft is inserted into the abdomen. A suture is placed at the distal margin of conduit to facilitate graft orientation and handling. The anastomosis between the SMV and the interposition graft is performed first, using two half-running sutures as described for type 3 vein reconstruction. Next, splenic and portal veins are crossclamped, the SMV is divided near the spleno-mesenteric junction, the graft is trimmed at the appropriate length, and the second anastomosis is fashioned using the same technique described above (Fig. 10) (video 4).
Type 4 vein resection and reconstruction. When resection involves the proximal portion of the superior mesenteric vein, portal flow can be maintained through the splenic vein (arrows). From Boggi U (Ed.) Minimally Invasive Surgery of the Pancreas. Springer-Verlag Italia s.r.l. 2018 – https://doi.org/10.1007/978-88-470-3958-2
Type 4 vein resection and reconstruction. a. The specimen was detached from all retroperitoneal attachments but the area of tumor adhesion to the superior mesenteric vein; b. Proximal anastomosis between the interposition graft and the superior mesenteric vein; c. The interposition graft is trimmed in preparation for the distal anastomosis; d. Vascular reconstruction completed
Anticoagulant prophylaxis and postoperative surveillance
Anticoagulation prophylaxis was not increased because of vein resection and reconstruction. According to our standard protocol, patients receiving chronic anti-aggregant or anticoagulant therapies are converted to low-molecular-weight heparin (LMWE) the week before surgery. The remaining patients receive the first dose of LMWE approximately 8 h before surgery. Postoperatively, LMWE is maintained for 4 weeks. No chronic anticoagulant or anti-aggregant therapy is prescribed only because of vein reconstruction.
Learning curve for RPD with vein resection and reconstruction
Feasibility learning curve for RPD with vein resection and reconstruction was defined based on operative time and was assessed with cumulative sum (CUSUM) analysis. The turning point of operative time curvature was used as a cut-off to compare 90-day mortality before and after completion of the feasibility learning curve. The ODD ratio was considered appropriate as measure of the effect size. The level of significance was set at p < 0.05.
Results
Between October 2008 and November 2021, a total of 783 pancreatoduodenectomies were performed at the Division of General and Transplant Surgery of the University of Pisa. Vein resection and reconstruction was performed in 233 patients (29.7%). Two hundred and fifty-six of these patients underwent RPD (32.6%), including 36 RPDs-VR (14.0%). Overall, RPD-VR was performed in 4.6% of all patients undergoing pancreatoduodenectomy, and in 15.4% of those requiring a vein resection. Concerning the type of vein resection, 4 were type 1 (11.1%), 10 were type 2 (27.8%), 12 were type 3 (33.3%) and 10 were type 4 (27.8%). Vascular patches used to repair type 2 resections were made of peritoneum (n = 8), greater saphenous vein (n = 1), and deceased donor aorta (n = 1). Interposition grafts used in type 4 resections were internal left jugular vein (n = 8), venous graft from deceased donor (n = 1) and spiral saphenous vein graft (n = 1).
Only one patient (2.8%) underwent conversion at the end of the procedure because of diffuse bleeding in a patient with previous bone marrow transplant and multiple comorbidities.
Mean operative time was 610 ± 85 min, with a median estimated blood loss of 878 ml (IQR: 680.4 -1430.2). Mean SMV/PV clamping time was 27.4 ± 17.6 min (type 1: 13.5 ± 1.3 min; type 2: 17.8 ± 1.4; minutes; type 3: 25.4 ± 1.7 min; type 4: 45.1 ± 5.3 min).
Ninety-day mortality was 8.3%. Excluding one death occurred during the learning curve, (procedure number 23), 90-day operative mortality was 5.5%. Both patients died due to delayed extraluminal hemorrhage. The first patient bleed from the SMA, without evidence of leak or pancreatitis. We speculate that hemorrhage resulted from too “deep” arterial divestment. The second patient developed a biliary leak, leading to erosion of both hepatic artery and SMV/PV. When the 36 RPD-VRs were divided in two groups of 18 procedures each, there were two deaths in the early experience (11.1%) and one thereafter (5.5%). However, the difference was not statistically significant (p = 1, Fisher exact test). Mortality at 90 days for contemporary, unmatched, OPD-VR was 5.1%.
There was 1 (2.8%) partial thrombosis of the reconstructed SMV/PV, treated with heparin infusion. One patient required repeat surgery due to “sentinel bleeding” secondary to partial erosion of a peritoneal patch caused by grade B postoperative pancreatic fistula.
Feasibility learning curve for RPD-VR was completed after 6 procedures (operative time declined from a median of 725 min [596.3–741.3] to 600 min [540–632.5]; p 0.027 (Fig. 11). After this turning point 90-day mortality declined from 33.3% to 3.3% [OR 14.5 (1.06–198.81); p = 0.039].
Discussion
Patients undergoing RPD-VR at our institution were highly selected. MIPD with vein resection and reconstruction is a demanding procedure that should be accurately planned and carefully performed. Unexpected vascular involvement is among the leading causes of conversion to open surgery in MIPD [34, 35], and injury to the superior mesenteric vessels has been associated with few perioperative deaths [36, 37].
Approximately 30% of the patients diagnosed with pancreatic cancer have a borderline resectable tumor [38]. Following neoadjuvant treatments, if there is no disease progression and Ca 19.9 has dropped below 50% of the pretreatment level [39], borderline resectable pancreatic tumors should be resected en-bloc with the involved vein segment. However, in some patients, vascular involvement is discovered only during surgery [40]. These patients usually do not have major vein involvement and vascular resection and reconstruction can be performed through a minimally invasive approach. Therefore, surgeons willing to pursue MIPD should be aware of the need to manage unexpected vein involvement. If the surgeon does not feel comfortable in proceeding to RPD-VR, conversion to open surgery is required. Trying to spare a vascular resection by insisting in difficult perivascular dissections, could result in positive resection margins or could lead to massive hemorrhage requiring emergency conversion to open surgery. In a collaborative study, conversion to open surgery was required in 65 of 709 MIPDs (9.1%). Vascular involvement was the leading cause of conversion (18/65; 27.7%). Overall, there were 12 emergency conversions (18,4%). Emergency conversion was associated with increased intraoperative blood loss and a higher blood transfusion rate [34].
Few preliminary experiences showed that MIPD with vein resection and reconstruction is feasible and that side-wall resections are frequently employed [4,5,6,7,8,9]. However, conversion to open surgery may be required in as many as one third of the patients [41], and only few comparative studies have been published. Beane and coworkers compared 50 RPD-VR to 330 unmatched RPDs. RPD-VR had greater mean preoperative Ca 19.9, higher prevalence of pancreatic cancer, firmer gland texture, and more frequent administration of neoadjuvant therapies. Postoperative outcomes were similar, but there was a trend toward greater 90-day mortality in RPD-VR (8.0% versus 2.8%) [4]. Marino et al. compared 10 RPDs-VR to 73 unmatched RPDs. At the baseline the two groups differed for the more frequent delivery of neoadjuvant treatments in RPD-VR. RPD was associated with longer operative time, higher blood loss, and more frequent use of blood transfusions. Ninety-day mortality was comparable [42]. Jin and coworkers compared 14 RPDs-VR to 70 OPDs with vein resection. The two groups were comparable at the baseline and perioperative results were equivalent. However, 90-day mortality in RPD-VR was 7.1% (versus 4.3% in OPD) [43]. Yang and coworkers provided a propensity matched comparison between 13 MIPDs with vein resection (including 6 RPDs-VR) and 13 OPDs with vein resection. The only difference was longer operative time in MIPD (720 vs. 485 min). It is worth to note that 5 MIPDs required open vascular reconstruction and that 2 laparoscopic MIPDs had a robotic vascular reconstruction (53.8%). Only type 1 procedures were completed by the initial minimally invasive approach [44]. These data demonstrate the need for further studies, as already proposed by the Miami guidelines on minimally invasive pancreas resection [45]. While it could be still too early to plan for a randomized controlled trial, registry analysis should be the next step in providing meaningful comparison with the open procedure.
RPD-VR is a formidable operation raising the question of who should be allowed to do it. The starting point, is the annual volume threshold of 20 MIPDs recommended by the Miami international guidelines [45]. Considering that approximately 30–40% of patients may be eligible for MIPD, it appears that centers willing to implement RPD should perform an average of 60 PDs per year [46]. Despite only some 20% of the centers reach this cutoff [47], recent evidence confirms that ≥ 60 pancreatic resections are required to qualify as a high volume center and that results further improve when this yearly number of procedures is met [48]. It is therefore wise to advice implementation of RPD only at high volume centers that have reached clinical excellence in OPD. The need for prudent implementation of RPD-VR is further reinforced by the lack of comparative studies with the open procedure, and by the fact that only few centers have performed a good number of RPDs. A recent review demonstrated that up to march 2021 only 28 centers had reported on RPD [49]. Number of PDs per center ranged from 6 to 500. Eighteen centers reported ≥ 37 procedures, and therefore completed the initial learning curve [50, 51]. Only six centers reached the threshold of 100 RPDs, that was associated with truly improved postoperative outcomes [52]. Finally, despite the lack of studies on the impact of individual surgeon volume in RPD, experience with OPD shows that also surgeon volume is associated with improved outcomes [53]. Therefore, we strongly recommend that RPD-VR is implemented in centers with an annual volume ≥ 20 RPDs, following completion of the learning curve. In this context, RPD-VR should be performed by expert surgeons.
In this study, completion of feasibility learning curve for RPD-VR was achieved after 6 procedures and was associated with improved 90-day mortality. However, these figures should be carefully interpreted in light of some limitations. First, before performing the first RPD-VR we had already performed 22 RPD and 63 robotic pancreatic resections. Second, our group has one of the largest world experiences with vascular resection and reconstruction in open pancreatectomy. Third, we are also transplant surgeons. Fourth, we had contemporary practice with other robotic operations requiring intracorporeal vascular anastomosis. Therefore, the generalizability of these results remains to be established.
From a technical point of view not all vein resections are the same. Wedge resections are not expected to increase postoperative morbidity as opposed to segmental resections [54]. Type 1 resections are just minor variations of the standard technique. In MIPD the majority of type 1 resections are performed using a vascular stapler [4].
In OPD, vein reconstruction can be readily accomplished in most patients irrespective of resection type. In MIPD, vein reconstruction following segmental vein resection adds further complexity. First, the bowel cannot be fully mobilized and pushed towards the liver. In addition, the patient is in a reverse Trendelenburg position. Second, when using a jump graft, the surgeon must consider that at the end of the procedure the reverse Trendelenburg position will be abolished. This could create a vascular kinking, if the interposition graft was too long. Third, vascular control can be troublesome when the SMV is involved near the mesenteric root. In these patients, once the vein has been divided, repositioning the bulldog clamp on the SMV may be extremely difficult. Fourth, the entire team must be ready for emergency conversion in case of uncontrolled bleeding. Emergency conversion in robotic surgery should occur according to a standardized protocol [55]. Fifth, if the vein segment to be resected includes the spleno-mesenteric junction, management of the splenic vein creates additional challenges.
Our experience shows that segmental vein resection and reconstruction is feasible in RPD. However, in patients with tumor abutment ≤ 180° needing type 4 resections including the spleno-mesenteric junction a large type 2 vein resection and reconstruction could be preferable. In these patients, arterial patches would be the ideal choice because they improve exposure during the suture and provide good support to the reconstructed vein, potentially preventing angulation and collapse. Of course, availability of an arterial graft is a major limiting factor, especially when vein resection is not planned. Fresh deceased donor graft may be available only at transplant centers. Cryopreserved grafts can be ordered from a tissue bank, but usually not an urgent basis.
In our experience complexity of RPD-VR was shown by a mean operative time approaching 10 h and a median estimated blood loss of approximately 900 ml. Despite we have not provided a matched comparison with contemporary RPD, these figures exceed those that we have published for standard RPD [16, 17, 20]. Operative time and blood loss are well established quality metrics in PD [56]. In the best scenario, RPD-VR could achieve the same postoperative results of RPD. More realistically, RPD-VR is expected to increase the risk of postoperative complications when compared to RPD. The key question, however, is whether the burden of postoperative complications following RPD-VR is increased when compared to OPD-VR. This is question cannot be currently answered.
As already reported for both renal and pancreatic robotic transplants [57, 58], the use e-PFTE for running vascular sutures is associated with several potential advantages. e-PFTE is a microporous vascular suture, mostly used in heart surgery, armed with a needle of the exact same size of the suture [59]. e-PTFE has no memory, good sliding properties [60], and elicits minimal tissue reaction [61]. When handled by robotic needle drivers, e-PTFE shows no loss in strength after repetitive manipulations, whereas polypropylene is weakened when touched three times at the same point [62]. In robotic sutures, maximal failure force of polypropylene is reduced by 35% as compared with 3% for ePTFE [63].
Following OPD with SMV/PV resection, benchmark rates of portal vein thrombosis and occlusion at hospital discharge are ≤ 14% and ≤ 4%, respectively [64]. A recent study demonstrates that the risk of thrombosis is higher in type 4 vein resection and reconstruction [65]. In RPD-VR reported rates of SMV/PV stenosis/thrombosis range between 7 and 9% [4, 43]. Therefore, the risk of SMV/PV thrombosis raises the important question on how to manage anticoagulation in these patients. Unfortunately, there is no agreed reply to this important question. The AHPBA guidelines for managing venous thromboembolism prophylaxis and anticoagulation for pancreatic surgery state that “data for anticoagulation after reconstruction is inconclusive and support for perioperative chemoprophylaxis with pancreatic surgery is similarly limited”. The final comment is:”the recommendation of anticoagulation after vascular reconstruction in pancreatic resection is weak (grade of recommendation, weak 2B)” [66]. In this study we have reported one partial SMV/PV thrombosis. In OPD, we have reported no SMV/PV occlusion with an incidence of non-occlusive thrombosis of 1.8% [12]. We speculate that this low rate of vascular thrombosis is the combined result of careful surgical technique plus standard anticoagulation prophylaxis. Clearly, more studies are needed to define the type of anticoagulant prophylaxis that should be used following pancreatoduodenectomy with SMV/PV resection and reconstruction.
In conclusion, the technique of RPD-VR requires some adaptations when compared to the open procedure. Here we have presented the tips and tricks implemented in 36 RPD-VR. Some of them were borrowed from OPD. Other technical details were imported from our robotic experience with renal and pancreatic transplantation. We suggest that surgeons willing to pursue RPD-VR should have a sound experience in OPD with vein resection and reconstruction. Hopefully, the tips and tricks provided in this study will facilitate safer implementation of RPD-VR.
Abbreviations
- e-PTFE:
-
Expanded polytetrafluoroethylene
- ISGPS:
-
International study group of pancreatic surgery
- LMWE:
-
Low-molecular-weight heparin
- MIPD:
-
Minimally invasive pancreatoduodenectomy
- OPD:
-
Open pancreatoduodenectomy
- RPD:
-
Robotic pancreatoduodenectomy
- RPD-VR:
-
Robotic pancreatoduodenectomy with vein resection
- SMA:
-
Superior mesenteric artery
- SMV:
-
Superior mesenteric vein
- SMV/PV:
-
Superior mesenteric/portal vein
References
Tempero MA, Malafa MP, Behrman SW et al (2014) Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 12:1083–1093. https://doi.org/10.6004/jnccn.2014.0106
Bockhorn M, Uzunoglu FG, Adham M et al (2014) Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 155:977–988. https://doi.org/10.1016/j.surg.2014.02.001
Tanaka M, Ito H, Ono Y, Matsueda K, Mise Y, Ishizawa T, Inoue Y, Takahashi Y, Hiratsuka M, Unno T, Saiura A (2019) Impact of portal vein resection with splenic vein reconstruction after pancreatoduodenectomy on sinistral portal hypertension: who needs reconstruction? Surgery 165:291–297. https://doi.org/10.1016/j.surg.2018.08.025
Beane JD, Zenati M, Hamad A, Hogg ME, Zeh HJ 3rd, Zureikat AH (2019) Robotic pancreatoduodenectomy with vascular resection: outcomes and learning curve. Surgery 166:8–14. https://doi.org/10.1016/j.surg.2019.01.037
Giulianotti PC, Addeo P, Buchs NC, Ayloo SM, Bianco FM (2011) Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas 40:1264–1270. https://doi.org/10.1097/MPA.0b013e318220e3a4
Kauffmann EF, Napoli N, Menonna F, Vistoli F, Amorese G, Campani D, Pollina LE, Funel N, Cappelli C, Caramella D, Boggi U (2016) Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg 401:1111–1122. https://doi.org/10.1007/s00423-016-1499-8
Kauffmann EF, Napoli N, Cacace C, Menonna F, Vistoli F, Amorese G, Boggi U (2020) Resection or repair of large peripancreatic arteries during robotic pancreatectomy. Updates Surg 72:145–153. https://doi.org/10.1007/s13304-020-00715-8
Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML (2015) Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg. https://doi.org/10.1007/s11605-014-2644-8
Khatkov IE, Izrailov RE, Khisamov AA, Tyutyunnik PS, Fingerhut A (2017) Superior mesenteric-portal vein resection during laparoscopic pancreatoduodenectomy. Surg Endosc 31:1488–1495. https://doi.org/10.1007/s00464-016-5115-3
Stefanidis D, Wang F, Korndorffer JR Jr, Dunne JB, Scott DJ (2010) Robotic assistance improves intracorporeal suturing performance and safety in the operating room while decreasing operator workload. Surg Endosc 24:377–382. https://doi.org/10.1007/s00464-009-0578-0
Zhang J, Qian HG, Leng JH, Cui M, Qiu H, Zhou GQ, Wu JH, Yang Y, Hao CY (2009) Long mesentericoportal vein resection and end-to-end anastomosis without graft in pancreaticoduodenectomy. J Gastrointest Surg 13:1524–1528. https://doi.org/10.1007/s11605-008-0777-3
Boggi U, Del Chiaro M, Croce C, Vistoli F, Signori S, Moretto C, Amorese G, Mazzeo S, Cappelli C, Campani D, Mosca F (2009) Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery 146:869–881. https://doi.org/10.1016/j.surg.2009.04.029
Napoli N, Kauffmann E, Cacace C, Menonna F, Caramella D, Cappelli C, Campani D, Cacciato Insilla A, Vasile E, Vivaldi C, Fornaro L, Amorese G, Vistoli F, Boggi U (2021) Factors predicting survival in patients with locally advanced pancreatic cancer undergoing pancreatectomy with arterial resection. Updates Surg 73:233–249. https://doi.org/10.1007/s13304-020-00883-7
Boggi U, Truty M, Zyromski NJ (2022) 2021 SSAT Debate: selective approach to resection of the superior mesenteric artery in pancreatic cancer vs superior mesenteric artery encasement is not an absolute contraindication for surgery in pancreatic cancer. J Gastrointest Surg. https://doi.org/10.1007/s11605-021-05237-1
Pietrabissa A, Moretto C, Boggi U, Di Candio G, Mosca F (2004) Laparoscopic distal pancreatomy: are we ready for a standardized technique? Semin Laparosc Surg 11:179–183. https://doi.org/10.1177/107155170401100307
Boggi U, Signori S, De Lio N, Perrone VG, Vistoli F, Belluomini M, Cappelli C, Amorese G, Mosca F (2013) Feasibility of robotic pancreaticoduodenectomy. Br J Surg 100:917–925. https://doi.org/10.1002/bjs.9135
Napoli N, Kauffmann EF, Menonna F, Perrone VG, Brozzetti S, Boggi U (2016) Indications, technique, and results of robotic pancreatoduodenectomy. Updates Surg 68:295–305. https://doi.org/10.1007/s13304-016-0387-7
Nagakawa Y, Nakata K, Nishino H et al (2022) International expert consensus on precision anatomy for minimally invasive pancreatoduodenectomy: PAM-HBP surgery project. J Hepatobiliary Pancreat Sci 29:124–135. https://doi.org/10.1002/jhbp.1081
Mazzeo S, Cappelli C, Caramella D, Del Chiaro M, Campani D, Pollina L, Caproni G, Battaglia V, Belcari A, Funel N, Di Candio G, Forasassi F, Boggi U, Bevilacqua G, Mosca F, Bartolozzi C (2007) Evaluation of vascular infiltration in resected patients for pancreatic cancer: comparison among multidetector CT, intraoperative findings and histopathology. Abdom Imaging 32:737–742. https://doi.org/10.1007/s00261-006-9172-2
Kauffmann EF, Napoli N, Ginesini M, Gianfaldoni C, Asta F, Salamone A, Amorese G, Vistoli F, Boggi U (2022) Feasibility of “cold” triangle robotic pancreatoduodenectomy. Surg Endosc. https://doi.org/10.1007/s00464-022-09411-7
Tee MC, Krajewski AC, Groeschl RT, Farnell MB, Nagorney DM, Kendrick ML, Cleary SP, Smoot RL, Croome KP, Truty MJ (2018) Indications and perioperative outcomes for pancreatectomy with arterial resection. J Am Coll Surg 227:255–269. https://doi.org/10.1016/j.jamcollsurg.2018.05.001
Garnier J, Traversari E, Ewald J, Marchese U, Delpero JR, Turrini O (2021) Venous reconstruction during pancreatectomy using polytetrafluoroethylene grafts: a single-center experience with standardized perioperative management. Ann Surg Oncol 28:5426–5433. https://doi.org/10.1245/s10434-021-09716-2
Cusack JC Jr, Fuhrman GM, Lee JE, Evans DB (1994) Managing unsuspected tumor invasion of the superior mesenteric-portal venous confluence during pancreaticoduodenectomy. Am J Surg 168:352–354. https://doi.org/10.1016/s0002-9610(05)80164-3
Chiu KM, Chu SH, Chen JS, Li SJ, Chan CY, Chen KS (2007) Spiral saphenous vein graft for portal vein reconstruction in pancreatic cancer surgery. Vasc Endovascular Surg 41:149–152. https://doi.org/10.1177/1538574406297259
Kleive D, Berstad AE, Verbeke CS, Haugvik SP, Gladhaug IP, Line PD, Labori KJ (2016) Cold-stored cadaveric venous allograft for superior mesenteric/portal vein reconstruction during pancreatic surgery. HPB (Oxford) 18:615–622. https://doi.org/10.1016/j.hpb.2016.05.010
Ono Y, Takahashi Y, Tanaka M, Matsueda K, Hiratsuka M, Inoue Y, Ito H, Saiura A (2021) Sinistral portal hypertension prediction during pancreatoduodenectomy with splenic vein resection. J Surg Res 259:509–515. https://doi.org/10.1016/j.jss.2020.10.005
Lee DY, Mitchell EL, Jones MA, Landry GJ, Liem TK, Sheppard BC, Billingsley KG, Moneta GL (2010) Techniques and results of portal vein/superior mesenteric vein reconstruction using femoral and saphenous vein during pancreaticoduodenectomy. J Vasc Surg 51:662–666. https://doi.org/10.1016/j.jvs.2009.09.025
Turley RS, Peterson K, Barbas AS, Ceppa EP, Paulson EK, Blazer DG 3rd, Clary BM, Pappas TN, Tyler DS, McCann RL, White RR (2012) Vascular surgery collaboration during pancreaticoduodenectomy with vascular reconstruction. Ann Vasc Surg 26:685–692. https://doi.org/10.1016/j.avsg.2011.11.009
Kubota K, Makuuchi M, Sugawara Y, Midorikawa Y, Sakamoto Y, Takayama T, Harihara Y (1998) Reconstruction of the hepatic and portal veins using a patch graft from the right ovarian vein. Am J Surg 176:295–197. https://doi.org/10.1016/s0002-9610(98)00149-4
Dokmak S, Aussilhou B, Sauvanet A, Nagarajan G, Farges O, Belghiti J (2015) Parietal peritoneum as an autologous substitute for venous reconstruction in hepatopancreatobiliary surgery. Ann Surg 262:366–371. https://doi.org/10.1097/SLA.0000000000000959
Shao Y, Yan S, Zhang QY, Shen Y, Zhang M, Wang WL, Zheng SS (2018) Autologous falciform ligament graft as a substitute for mesentericoportal vein reconstruction in pancreaticoduodenectomy. Int J Surg 53:159–162. https://doi.org/10.1016/j.ijsu.2018.03.045
Starzl TE, Iwatsuki S, Shaw BW Jr (1984) A growth factor in fine vascular anastomoses. Surg Gynecol Obstet 159:164–165
Irie S, Yoshioka R, Imamura H, Ono Y, Sato T, Inoue Y, Ito H, Mise Y, Takahashi Y, Saiura A (2022) Parachute technique for portal vein reconstruction during pancreaticoduodenectomy with portal vein resection in patients with pancreatic head cancer. Langenbecks Arch Surg 407:383–389. https://doi.org/10.1007/s00423-021-02338-y
Lof S, Vissers FL, Klompmaker S, Berti S, Boggi U, Coratti A, Dokmak S, Fara R, Festen S, D’Hondt M, Khatkov I, Lips D, Luyer M, Manzoni A, Rosso E, Saint-Marc O, Besselink MG, Abu Hilal M, European consortium on Minimally Invasive Pancreatic Surgery (E-MIPS) (2021) Risk of conversion to open surgery during robotic and laparoscopic pancreatoduodenectomy and effect on outcomes: international propensity score-matched comparison study. Br J Surg 108:80–87. https://doi.org/10.1093/bjs/znaa026
Lof S, Korrel M, van Hilst J, Moekotte AL, Bassi C, Butturini G, Boggi U, Dokmak S, Edwin B, Falconi M, Fuks D, de Pastena M, Zerbi A, Besselink MG, Abu Hilal M, European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS) (2021) Outcomes of elective and emergency conversion in minimally invasive distal pancreatectomy for pancreatic ductal adenocarcinoma: an international multicenter propensity score-matched study. Ann Surg 274:e1001–e1007. https://doi.org/10.1097/SLA.0000000000003717
Chalikonda S, Aguilar-Saavedra JR, Walsh RM (2012) Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc 26:2397–2402. https://doi.org/10.1007/s00464-012-2207-6
van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MG, Gerhards MF, de Hingh IH, Karsten TM, Lips DJ, Luyer MD, Busch OR, Festen S, Besselink MG, Dutch Pancreatic Cancer Group (2019) Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 4:199–207. https://doi.org/10.1016/S2468-1253(19)30004-4
Kamisawa T, Wood LD, Itoi T, Takaori K (2016) Pancreatic cancer. Lancet 388(10039):73–85. https://doi.org/10.1016/S0140-6736(16)00141-0
Reni M, Zanon S, Balzano G, Nobile S, Pircher CC, Chiaravalli M, Passoni P, Arcidiacono PG, Nicoletti R, Crippa S, Slim N, Doglioni C, Falconi M, Gianni L (2017) Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann Oncol 28:2786–2792. https://doi.org/10.1093/annonc/mdx495
Kim PT, Wei AC, Atenafu EG, Cavallucci D, Cleary SP, Moulton CA, Greig PD, Gallinger S, Serra S, McGilvray ID (2013) Planned versus unplanned portal vein resections during pancreaticoduodenectomy for adenocarcinoma. Br J Surg 100:1349–1356. https://doi.org/10.1002/bjs.9222
Shyr BU, Chen SC, Shyr YM, Wang SE (2020) Surgical, survival, and oncological outcomes after vascular resection in robotic and open pancreaticoduodenectomy. Surg Endosc 34:377–383. https://doi.org/10.1007/s00464-019-06779-x
Marino MV, Giovinazzo F, Podda M, Gomez Ruiz M, Gomez Fleitas M, Pisanu A, Latteri MA, Takaori K (2020) Robotic-assisted pancreaticoduodenectomy with vascular resection. Description of the surgical technique and analysis of early outcomes. Surg Oncol 35:344–350. https://doi.org/10.1016/j.suronc.2020.08.025
Jin J, Yin SM, Weng Y, Chen M, Shi Y, Ying X, Gemenetzis G, Qin K, Zhang J, Deng X, Peng C, Shen B (2022) Robotic versus open pancreaticoduodenectomy with vascular resection for pancreatic ductal adenocarcinoma: surgical and oncological outcomes from pilot experience. Langenbecks Arch Surg 407:1489–1497. https://doi.org/10.1007/s00423-021-02364-w
Yang E, Chong Y, Wang Z, Koh YX, Lim KI, Goh P, BK, (2022) Minimally-invasive versus open pancreatoduodenectomies with vascular resection: a 1:1 propensity-matched comparison study. J Minim Access Surg 18:420–425. https://doi.org/10.4103/jmas.jmas_201_21
Asbun HJ, Moekotte AL, Vissers FL et al (2020) The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 271:1–14. https://doi.org/10.1097/SLA.0000000000003590
Jones LR, Zwart MJW, Molenaar IQ, Koerkamp BG, Hogg ME, Hilal MA, Besselink MG (2020) Robotic pancreatoduodenectomy: patient selection, volume criteria, and training programs. Scand J Surg 109:29–33. https://doi.org/10.1177/1457496920911815
Swanson RS, Pezzi CM, Mallin K, Loomis AM, Winchester DP (2014) The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the national cancer data base. Ann Surg Oncol 21:4059–4067. https://doi.org/10.1245/s10434-014-4036-4
Balzano G, Guarneri G, Pecorelli N, Paiella S, Rancoita PMV, Bassi C, Falconi M (2020) Modelling centralization of pancreatic surgery in a nationwide analysis. Br J Surg 107:1510–1519. https://doi.org/10.1002/bjs.11716
Levi Sandri GB, Abu Hilal M, Dokmak S, Edwin B, Hackert T, Keck T, Khatkov I, Besselink MG, Boggi U, Innovation E-AHPBA, Committee D (2022) Figures do matter: a literature review of 4587 robotic pancreatic resections and their implications on training. J Hepatobiliary Pancreat Sci. https://doi.org/10.1002/jhbp.1209
Fung G, Sha M, Kunduzi B, Froghi F, Rehman S, Froghi S (2022) Learning curves in minimally invasive pancreatic surgery: a systematic review. Langenbecks Arch Surg. https://doi.org/10.1007/s00423-022-02470-3
Chan KS, Wang ZK, Syn N, Goh BKP (2021) Learning curve of laparoscopic and robotic pancreas resections: a systematic review. Surgery 170:194–206. https://doi.org/10.1016/j.surg.2020.11.046
Shi Y, Wang W, Qiu W, Zhao S, Wang J, Weng Y, Huo Z, Jin J, Wang Y, Deng X, Shen B, Peng C (2021) Learning curve from 450 cases of robot-assisted pancreaticoduocectomy in a high-volume pancreatic center: optimization of operative procedure and a retrospective study. Ann Surg 274:e1277–e1283. https://doi.org/10.1097/SLA.0000000000003664
Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL (2003) Surgeon volume and operative mortality in the United States. N Engl J Med 349:2117–2127
Groen JV, Michiels N, van Roessel S, Besselink MG, Bosscha K, Busch OR, van Dam R, van Eijck CHJ, Koerkamp BG, van der Harst E, de Hingh IH, Karsten TM, Lips DJ, de Meijer VE, Molenaar IQ, Nieuwenhuijs VB, Roos D, van Santvoort HC, Wijsman JH, Wit F, Zonderhuis BM, de Vos-Geelen J, Wasser MN, Bonsing BA, Stommel MWJ, Mieog JSD, Dutch Pancreatic Cancer Group (2021) Venous wedge and segment resection during pancreatoduodenectomy for pancreatic cancer: impact on short- and long-term outcomes in a nationwide cohort analysis. Br J Surg 109:96–104. https://doi.org/10.1093/bjs/znab345
O’Sullivan OE, O’Sullivan S, Hewitt M, O’Reilly BA (2016) Da Vinci robot emergency undocking protocol. J Robot Surg 10:251–253. https://doi.org/10.1007/s11701-016-0590-z
Ball CG, Pitt HA, Kilbane ME, Dixon E, Sutherland FR, Lillemoe KD (2010) Peri-operative blood transfusion and operative time are quality indicators for pancreatoduodenectomy. HPB (Oxford) 12:465–471. https://doi.org/10.1111/j.1477-2574.2010.00209.x
Boggi U, Signori S, Vistoli F, D’Imporzano S, Amorese G, Consani G, Guarracino F, Marchetti P, Focosi D, Mosca F (2012) Laparoscopic robot-assisted pancreas transplantation: first world experience. Transplantation 93:201–206. https://doi.org/10.1097/TP.0b013e318238daec
Boggi U, Vistoli F, Signori S, D’Imporzano S, Amorese G, Consani G, Guarracino F, Melfi F, Mussi A, Mosca F (2011) Robotic renal transplantation: first European case. Transpl Int 24:213–218. https://doi.org/10.1111/j.1432-2277.2010.01191.x
Towler MA, Tribble CG, Pavlovich LJ, Milam JT, Morgan RF, Edlich RF (1993) Biomechanical performance of new vascular sutures and needles for use in polytetrafluoroethylene grafts. J Appl Biomater 4:87–95. https://doi.org/10.1002/jab.770040112
Cavallini M (2001) “Growth factor” technique using expanded polytetrafluoroethylene monofilament for arteriovenous fistulae. Surg Today 31:277–279. https://doi.org/10.1007/s005950170187
Setzen G, Williams EF 3rd (1997) Tissue response to suture materials implanted subcutaneously in a rabbit model. Plast Reconstr Surg 100:1788–1795. https://doi.org/10.1097/00006534-199712000-00023
Ricchiuti D, Cerone J, Shie S, Jetley A, Noe D, Kovacik M (2010) Diminished suture strength after robotic needle driver manipulation. J Endourol 24:1509–1513. https://doi.org/10.1089/end.2009.0573
Diks J, Nio D, Linsen MA, Rauwerda JA, Wisselink W (2007) Suture damage during robot-assisted vascular surgery: is it an issue? Surg Laparosc Endosc Percutan Tech 17:524–527. https://doi.org/10.1097/SLE.0b013e318150e590
Raptis DA, Sánchez-Velázquez P, Machairas N et al (2020) Defining benchmark outcomes for pancreatoduodenectomy with portomesenteric venous resection. Ann Surg 272:731–737. https://doi.org/10.1097/SLA.0000000000004267
Hackert T, Klaiber U, Hinz U, Strunk S, Loos M, Strobel O, Berchtold C, Kulu Y, Mehrabi A, Müller-Stich BP, Schneider M, Büchler MW (2022) Portal vein resection in pancreatic cancer surgery: risk of thrombosis and radicality determine survival. Ann Surg. https://doi.org/10.1097/SLA.0000000000005444
Clancy TE, Baker EH, Maegawa FA, Raoof M, Winslow E, House MG, Members of the AHPBA Professional Standards Committee (2022) AHPBA guidelines for managing VTE prophylaxis and anticoagulation for pancreatic surgery. HPB (Oxford) 24:575–585. https://doi.org/10.1016/j.hpb.2021.12.010
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This study did not receive funds or grants.
Author information
Authors and Affiliations
Contributions
EFK: conception and design of the study, drafting the article, acquisition of data, final approval of the version to be submitted. NN: acquisition of data, revising it critically, final approval of the version to be submitted. MG: acquisition of data, revising it critically, final approval of the version to be submitted. CG: acquisition of data, revising it critically, final approval of the version to be submitted. FA: acquisition of data, revising it critically, final approval of the version to be submitted. AS: acquisition of data, revising it critically, final approval of the version to be submitted. AR: acquisition of data, revising it critically, final approval of the version to be submitted. ADD: acquisition of data, revising it critically, final approval of the version to be submitted. FV: acquisition of data, revising it critically, final approval of the version to be submitted. GA: acquisition of data, revising it critically, final approval of the version to be submitted. UB: conception and design of the study, drafting the article, final approval of the version to be submitted.
Corresponding author
Ethics declarations
Disclosures
Emanuele F Kauffmann, Niccolò Napoli, Michael Ginesini, Cesare Gianfaldoni, Fabio Asta, Alice Salamone, Armando Di Dato, Allegra Ripolli, Fabio Vistoli, Gabriella Amorese, and Ugo Boggi have no conflicts of interest or financial to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 75123 kb)
Supplementary file2 (MP4 96918 kb)
Supplementary file3 (MP4 87007 kb)
Supplementary file4 (MP4 79545 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kauffmann, E.F., Napoli, N., Ginesini, M. et al. Tips and tricks for robotic pancreatoduodenectomy with superior mesenteric/portal vein resection and reconstruction. Surg Endosc 37, 3233–3245 (2023). https://doi.org/10.1007/s00464-022-09860-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09860-0