Abstract
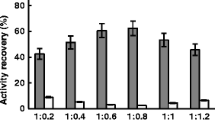
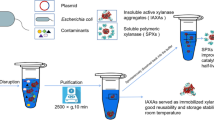
The combined cross-linked enzyme aggregates (combi-CLEAs) containing galactitol dehydrogenase (Gdh) and NADH oxidase (Nox) were prepared for l-tagatose synthesis. To prevent the excess consumption of cofactor, Nox in the combi-CLEAs was used to in situ regenerate NAD+. In the immobilization process, ammonia sulfate and glutaraldehyde were used as the precipitant and cross-linking reagent, respectively. The preparation conditions were optimized as follows: 60% ammonium sulfate, 1:1 (molar ratio) of Gdh to Nox, 20:1 (molar ratio) of protein to glutaraldehyde, and 6 h of cross-linking time at 35 °C. Under these conditions, the activity of the combi-CLEAs was 210 U g−1. The combi-CLEAs exhibited higher thermostability and preserved 51.5% of the original activity after eight cycles of reuses at 45 °C. The combi-CLEAs were utilized for the preparation of l-tagatose without by-products. Therefore, the combi-CLEAs have the industrial potential for the bioconversion of galactitol to l-tagatose.
Graphical abstract









Similar content being viewed by others
Data availability
The datasets used or analyzed in this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Roy S, Chikkerur J, Roy SC et al (2018) Tagatose as a potential nutraceutical: production, properties, biological roles, and applications. J Food Sci 83:2699–2709. https://doi.org/10.1111/1750-3841.14358
Oh D-K (2007) Tagatose: properties, applications, and biotechnological processes. Appl Microbiol Biotechnol 76:1–8. https://doi.org/10.1007/s00253-007-0981-1
Roh HJ, Kim P, Park YC et al (2000) Bioconversion of D-galactose into D-tagatose by expression of l-arabinose isomerase. Biotechnol Appl Biochem 31:1–4. https://doi.org/10.1042/ba19990065
Guerrero-Wyss M, Durán Agüero S, Angarita Dávila L (2018) D-tagatose is a promising sweetener to control glycaemia: a new functional food. BioMed Res Int 2018:1–7. https://doi.org/10.1155/2018/8718053
Espinosa I, Fogelfeld L (2010) Tagatose: from a sweetener to a new diabetic medication? Exp Opin Investig Drugs 19:285–294. https://doi.org/10.1517/13543780903501521
Patel SKS, Otari SV, Chan Kang Y, Lee J-K (2017) Protein–inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in l-xylulose production. RSC Adv 7:3488–3494. https://doi.org/10.1039/C6RA24404A
Mei W, Wang L, Zang Y et al (2016) Characterization of an L-arabinose isomerase from Bacillus coagulans NL01 and its application for D-tagatose production. BMC Biotechnol 16:55. https://doi.org/10.1186/s12896-016-0286-5
Soetedjo JNM, van de Bovenkamp HH, Deuss PJ et al (2017) Biobased furanics: kinetic studies on the acid catalyzed decomposition of 2-hydroxyacetyl furan in water using Brönsted acid catalysts. ACS Sustain Chem Eng 5:3993–4001. https://doi.org/10.1021/acssuschemeng.6b03198
Nath A, Verasztó B, Basak S et al (2015) Synthesis of lactose-derived nutraceuticals from dairy waste whey—a review. Food Bioprocess Technol 9:16–48. https://doi.org/10.1007/s11947-015-1572-2
Drabo P, Delidovich I (2018) Catalytic isomerization of galactose into tagatose in the presence of bases and Lewis acids. Catal Commun 107:24–28. https://doi.org/10.1016/j.catcom.2018.01.011
Kim P (2004) Current studies on biological tagatose production using l-arabinose isomerase: a review and future perspective. Appl Microbiol Biotechnol 65:243–249. https://doi.org/10.1007/s00253-004-1665-8
Kohlmeier MG, Bailey-Elkin BA, Mark BL et al (2021) Characterization of the sorbitol dehydrogenase SmoS from Sinorhizobium meliloti 1021. Acta Crystallogr Sect D 77:380–390. https://doi.org/10.1107/S2059798321001017
Wang X, Yiu H (2016) Heterogeneous catalysis mediated cofactor NADH regeneration for enzymatic reduction. ACS Catal 6:1880–1886. https://doi.org/10.1021/acscatal.5b02820
Hwang ET, Lee S (2019) Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal 9:4402–4425. https://doi.org/10.1021/acscatal.8b04921
Wang X, Saba T, Yiu HHP et al (2017) Cofactor NAD(P)H regeneration inspired by heterogeneous pathways. Chem 2:621–654. https://doi.org/10.1016/j.chempr.2017.04.009
Demir AS, Talpur FN, Betul Sopaci S et al (2011) Selective oxidation and reduction reactions with cofactor regeneration mediated by galactitol-, lactate-, and formate dehydrogenases immobilized on magnetic nanoparticles. J Biotechnol 152:176–183. https://doi.org/10.1016/j.jbiotec.2011.03.002
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307. https://doi.org/10.1002/adsc.200700082
Cui JD, Jia SR (2015) Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: current development and future challenges. Crit Rev Biotechnol 35:15–28. https://doi.org/10.3109/07388551.2013.795516
Cui JD, Cui LL, Zhang SP et al (2014) Hybrid magnetic cross-linked enzyme aggregates of phenylalanine ammonia lyase from rhodotorula glutinis. PLoS ONE 9:e97221. https://doi.org/10.1371/journal.pone.0097221
Cao L (2005) Immobilised enzymes: science or art? Curr Opin Chem Biol 9:217–226. https://doi.org/10.1016/j.cbpa.2005.02.014
Cao L, van Langen L, Sheldon RA (2003) Immobilised enzymes: carrier-bound or carrier-free? Curr Opin Biotechnol 14:387–394. https://doi.org/10.1016/s0958-1669(03)00096-x
Talekar S, Pandharbale A, Ladole M et al (2013) Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-CLEAs): a tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour Technol 147:269–275. https://doi.org/10.1016/j.biortech.2013.08.035
Ramos MD, Miranda LP, Giordano RLC et al (2018) 1,3-Regiospecific ethanolysis of soybean oil catalyzed by crosslinked porcine pancreas lipase aggregates. Biotechnol Prog 34:910–920. https://doi.org/10.1002/btpr.2636
Cui J, Cui L, Jia S et al (2016) Hybrid cross-linked lipase aggregates with magnetic nanoparticles: a robust and recyclable biocatalysis for the epoxidation of oleic acid. J Agric Food Chem 64:7179–7187. https://doi.org/10.1021/acs.jafc.6b01939
Cui J, Zhang S, Sun LM (2012) Cross-linked enzyme aggregates of phenylalanine ammonia lyase: novel biocatalysts for synthesis of l-phenylalanine. Appl Biochem Biotechnol 167:835–844. https://doi.org/10.1007/s12010-012-9738-0
Cui J, Zhao Y, Feng Y et al (2017) Encapsulation of spherical cross-linked phenylalanine ammonia lyase aggregates in mesoporous biosilica. J Agric Food Chem 65:618–625. https://doi.org/10.1021/acs.jafc.6b05003
Cui J, Zhao Y, Tan Z et al (2017) Mesoporous phenylalanine ammonia lyase microspheres with improved stability through calcium carbonate templating. Int J Biol Macromol 98:887–896. https://doi.org/10.1016/j.ijbiomac.2017.02.059
Xu M-Q, Li F-L, Yu W-Q et al (2020) Combined cross-linked enzyme aggregates of glycerol dehydrogenase and NADH oxidase for high efficiency in situ NAD+ regeneration. Int J Biol Macromol 144:1013–1021. https://doi.org/10.1016/j.ijbiomac.2019.09.178
Freimund S, Huwig A, Giffhorn F et al (1996) Convenient chemo-enzymatic synthesis of d-tagatose. J Carbohydr Chem 15:115–120. https://doi.org/10.1080/07328309608005430
Su W-B, Li F-L, Li X-Y et al (2021) Using galactitol dehydrogenase coupled with water-forming NADH oxidase for efficient enzymatic synthesis of L-tagatose. New Biotechnol 62:18–25. https://doi.org/10.1016/j.nbt.2021.01.003
Li F-L, Shi Y, Zhang J-X et al (2018) Cloning, expression, characterization and homology modeling of a novel water-forming NADH oxidase from Streptococcus mutans ATCC 25175. Int J Biol Macromol 113:1073–1079. https://doi.org/10.1016/j.ijbiomac.2018.03.016
Zhuang M-Y, Jiang X-P, Ling X-M et al (2018) Immobilization of glycerol dehydrogenase and NADH oxidase for enzymatic synthesis of 1,3-dihydroxyacetone with in situ cofactor regeneration. J Chem Technol Biotechnol 93:2351–2358. https://doi.org/10.1002/jctb.5579
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Lee D-W, Jang H-J, Choe E-A et al (2004) Characterization of a thermostable L-arabinose (D-galactose) isomerase from the hyperthermophilic eubacterium Thermotoga maritima. Appl Environ Microbiol 70:1397–1404. https://doi.org/10.1128/aem.70.3.1397-1404.2004
Mezzenga R, Jung J-M, Adamcik J (2010) Effects of charge double layer and colloidal aggregation on the isotropic-nematic transition of protein fibers in water. Langmuir 26:10401–10405. https://doi.org/10.1021/la101636r
Pchelintsev NA, Youshko MI, Švedas VK (2009) Quantitative characteristic of the catalytic properties and microstructure of cross-linked enzyme aggregates of penicillin acylase. J Mol Catal B Enzym 56:202–207. https://doi.org/10.1016/j.molcatb.2008.05.006
Aytar B, Bakir U (2008) Preparation of cross-linked tyrosinase aggregates. Process Biochem 43:125–131. https://doi.org/10.1016/j.procbio.2007.11.001
Nadar S, Muley A, Ladole M et al (2015) Macromolecular cross-linked enzyme aggregates (M-CLEAs) of α-amylase. Int J Biol Macromol 84:69–78. https://doi.org/10.1016/j.ijbiomac.2015.11.082
Xu M-Q, Wang S-S, Li L-N et al (2018) Combined cross-linked enzyme aggregates as biocatalysts. Catalysts 8:460. https://doi.org/10.3390/catal8100460
Talekar S, Joshi A, Joshi G et al (2013) Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv 3:12485–12511. https://doi.org/10.1039/C3RA40818C
Sheldon RA (2011) Cross-linked enzyme aggregates as industrial biocatalysts. Org Process Res Dev 15:213–223. https://doi.org/10.1021/op100289f
Mateo C, Palomo JM, van Langen LM et al (2004) A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol Bioeng 86:273–276. https://doi.org/10.1002/bit.20033
Arana-Peña S, Carballares D, Morellon-Sterlling R et al (2020) Enzyme co-immobilization: always the biocatalyst designers’ choice…or not? Biotechnol Adv 51:107584. https://doi.org/10.1016/j.biotechadv.2020.107584
Benítez-Mateos A, Nidetzky B, Bolivar J et al (2017) Single-particle studies to advance the characterization of heterogeneous biocatalysts. ChemCatChem 10:654–665. https://doi.org/10.1002/cctc.201701590
Wang M, Jia C, Qi W et al (2011) Porous-CLEAs of papain: application to enzymatic hydrolysis of macromolecules. Bioresour Technol 102:3541–3545. https://doi.org/10.1016/j.biortech.2010.08.120
Xu D-Y, Chen J-Y, Yang Z (2012) Use of cross-linked tyrosinase aggregates as catalyst for synthesis of l-DOPA. Biochem Eng J 63:88–94. https://doi.org/10.1016/j.bej.2011.11.009
Hanamoto JH, Dupuis P, El-Sayed MA (1984) On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci USA 81:7083–7087. https://doi.org/10.1073/pnas.81.22.7083
Wondrak EM, Louis JM, Oroszlan S (1991) The effect of salt on the Michaelis Menten constant of the HIV-1 protease correlates with the Hofmeister series. FEBS Lett 280:344–346. https://doi.org/10.1016/0014-5793(91)80327-Y
Zhen Q, Wang M, Qi W et al (2013) Preparation of β-mannanase CLEAs using macromolecular cross-linkers. Catal Sci Technol 3:1937–1941. https://doi.org/10.1039/C3CY20886A
Liu Y, Feng Y, Wang L et al (2019) Structural insights into phosphite dehydrogenase variants favoring a non-natural redox cofactor. ACS Catal 9:1883–1887. https://doi.org/10.1021/acscatal.8b04822
Goetze D, Foletto EF, da Silva HB et al (2017) Effect of feather meal as proteic feeder on combi-CLEAs preparation for grape juice clarification. Process Biochem 62:122–127. https://doi.org/10.1016/j.procbio.2017.07.015
Iyer PV, Ananthanarayan L (2008) Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem 43:1019–1032. https://doi.org/10.1016/j.procbio.2008.06.004
Rollini M, Manzoni M (2005) Bioconversion of d-galactitol to tagatose and dehydrogenase activity induction in Gluconobacter oxydans. Process Biochem 40:437–444. https://doi.org/10.1016/j.procbio.2004.01.028
Jørgensen F, Hansen O, Stougaard P (2004) Enzymatic conversion of d-galactose to d-tagatose: heterologous expression and characterisation of a thermostable l-arabinose isomerase from Thermoanaerobacter mathranii. Appl Microbiol Biotechnol 64:816–822. https://doi.org/10.1007/s00253-004-1578-6
Funding
The authors appreciated the financial support from the Natural Science Foundation of Guangxi Province (No. 2019GXNSFAA185059).
Author information
Authors and Affiliations
Contributions
Design of experiments: Y-WZ, X-YL, and M-QX; performance of experiment and drawing of graphs: M-QX, X-YL, and HL; manuscript draft and compilation: X-YL and Y-WZ; correction and revision of the manuscript: Y-WZ, QZ, and JG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
The authors are agreeable to the publication of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, XY., Xu, MQ., Liu, H. et al. Preparation of combined cross-linked enzyme aggregates containing galactitol dehydrogenase and NADH oxidase for l-tagatose synthesis via in situ cofactor regeneration. Bioprocess Biosyst Eng 45, 353–364 (2022). https://doi.org/10.1007/s00449-021-02665-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02665-w