Abstract
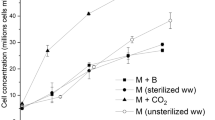
Synechocystis sp. has remarkable mixotrophic capabilities, as well as an efficient exploitation of nitrogen and phosphorus, that may be applied in wastewater treatment based on cyanobacteria. To better investigate the exploitation of algal mixotrophy in bioremediation, this species was used in axenic respirometric tests to ascertain the effect of high light and non limiting CO2 supply on the overall regulation of mixotrophy, resulting in an inhibition of the exploitation of organic carbon. The same species was then cultured in real, unsterilized effluent obtained from the acidogenic fermentation of sludge, which contains a high concentration of nutrients (approximately 600, 90 and 6000 mg L−1 of N, P and COD, respectively) and it is often inhibiting for many microalgal species. On the contrary, Synechocystis sp., showed a remarkable growth and a removal up to 96% of phosphorus, 66% of nitrogen and of 68% of COD in such a complex waste stream.






Similar content being viewed by others
References
Rawat I, Gupta SK, Shriwastav A, Singh P, Kumari S, Bux F (2016) Microalgae applications in wastewater treatment. In: Bux F, Chisti Y (eds) Algae biotechnology. Green energy and technology. Springer, Cham, pp 249–268
Lema JM, Bongards M, Chaparro A et al (2016) Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art and proposals for improvement. Appl Energy 179:1251–1268. https://doi.org/10.1016/j.apenergy.2016.07.043
Sforza E, Pastore M, Spagni A, Bertucco A (2018) Microalgae-bacteria gas exchange in wastewater: how mixotrophy may reduce the oxygen supply for bacteria. Environ Sci Pollut Res 25:28004–28014. https://doi.org/10.1007/s11356-018-2834-0
Percival SL, Williams DW (2014) Chapter Five - Cyanobacteria. In: Percival SL, Yates MV, Williams DW et al (eds) Microbiology of waterborne diseases, 2nd edn. Academic Press, London, pp 79–88
El-Bestawy E (2008) Treatment of mixed domestic-industrial wastewater using cyanobacteria. J Ind Microbiol Biotechnol 35:1503–1516. https://doi.org/10.1007/s10295-008-0452-4
Vijayakumar S (2012) Potential applications of cyanobacteria in industrial effluents—a review. J Bioremediation Biodegrad. https://doi.org/10.4172/2155-6199.1000154
Panda B, Mallick N (2007) Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett Appl Microbiol 44:194–198. https://doi.org/10.1111/j.1472-765X.2006.02048.x
Ansari S, Fatma T (2016) Cyanobacterial polyhydroxybutyrate (PHB): screening, optimization and characterization. PLoS One 11:1–20. https://doi.org/10.1371/journal.pone.0158168
Balaji S, Gopi K, Muthuvelan B (2013) A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res 2:278–285. https://doi.org/10.1016/j.algal.2013.03.002
Sheng J, Kim HW, Badalamenti JP et al (2011) Effects of temperature shifts on growth rate and lipid characteristics of Synechocystis sp. PCC6803 in a bench-top photobioreactor. Bioresour Technol 102:11218–11225. https://doi.org/10.1016/j.biortech.2011.09.083
Yu Y, You L, Liu D et al (2013) Development of Synechocystis sp. PCC 6803 as a phototrophic cell factory. Mar Drugs 11:2894–2916. https://doi.org/10.3390/md11082894
Rippka R, Deruelles JJBW, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiol SGM 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Bryant DA (ed) (1994) The molecular biology of cyanobacteria. Advances in photosynthesis and respiration, vol 1. Springer, Dordrecht, pp 559–579
Innamorati M, Ferrari I, Marino D, Ribera D’Alcala M (1990) Metodi nell’ ecologia del plancton marino. Nova Thalassia, vol 11. Ed Lindt, Trieste, pp 123–132
Frison N, Fatone F, Longo S et al (2014) Recovery of volatile fatty acids from fermentation of sewage sludge in municipal wastewater treatment plants. Bioresour Technol 175:436–444. https://doi.org/10.1016/j.biortech.2014.09.107
Basset N, Katsou E, Frison N et al (2016) Integrating the selection of PHA storing biomass and nitrogen removal via nitrite in the main wastewater treatment line. Bioresour Technol 200:820–829. https://doi.org/10.1016/j.biortech.2015.10.063
Decostere B, Janssens N, Alvarado A et al (2013) A combined respirometer-titrimeter for the determination of microalgae kinetics: experimental data collection and modelling. Chem Eng J 222:85–93. https://doi.org/10.1016/j.cej.2013.01.103
Rossi S, Bellucci M, Marazzi F et al (2018) Activity assessment of microalgal-bacterial consortia based on respirometric tests. Water Sci Technol 87(1–2):207–215
Sforza E, Pastore M, Barbera E, Bertucco A (2019) Respirometry as a tool to quantify kinetic parameters of microalgal mixotrophic growth. Bioprocess Biosys Eng. https://doi.org/10.1007/s00449-019-02087-9
Nguyen BT, Rittmann BE (2016) Effects of inorganic carbon and pH on growth kinetics of Synechocystis sp. PCC 6803. Algal Res 19:363–369. https://doi.org/10.1016/j.algal.2016.03.011
Sforza E, Cipriani R, Morosinotto T et al (2012) Excess CO2 supply inhibits mixotrophic growth of Chlorella protothecoides and Nannochloropsis salina. Bioresour Technol 104:523–529. https://doi.org/10.1016/j.biortech.2011.10.025
Thiel K, Vuorio E, Aro EM, Kallio PT (2017) The effect of enhanced acetate influx on Synechocystis sp. PCC 6803 metabolism. Microb Cell Fact 16:1–12. https://doi.org/10.1186/s12934-017-0640-x
Wan N, Abernathy M, Tang JKH et al (2015) Cyanobacterial photo-driven mixotrophic metabolism and its advantages for biosynthesis. Front Chem Sci Eng 9:308–316. https://doi.org/10.1007/s11705-015-1521-7
Chalima A, Oliver L, de Castro L et al (2017) Utilization of volatile fatty acids from microalgae for the production of high added value compounds. Fermentation 3:54. https://doi.org/10.3390/fermentation3040054
Liu CH, Chang CY, Liao Q et al (2013) Photoheterotrophic growth of Chlorella vulgaris ESP6 on organic acids from dark hydrogen fermentation effluents. Bioresour Technol 145:331–336. https://doi.org/10.1016/j.biortech.2012.12.111
Chiranjeevi P, Venkata Mohan S (2017) Diverse acidogenic effluents as feedstock for microalgae cultivation: dual phase metabolic transition on biomass growth and lipid synthesis. Bioresour Technol 242:191–196. https://doi.org/10.1016/j.biortech.2017.04.059
Yun YS, Park JM (2003) Kinetic modeling of the light-dependent photosynthetic activity of the green microalga Chlorella vulgaris. Biotechnol Bioeng 83:303–311. https://doi.org/10.1002/bit.10669
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors would like to acknowledge Dr. Nicola Frison, University of Verona for kindly providing the wastewater.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trentin, G., Bertucco, A. & Sforza, E. Mixotrophy in Synechocystis sp. for the treatment of wastewater with high nutrient content: effect of CO2 and light. Bioprocess Biosyst Eng 42, 1661–1669 (2019). https://doi.org/10.1007/s00449-019-02162-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02162-1