Abstract
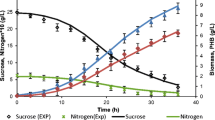
An integrated metabolic–polymerization–macroscopic model, describing the microbial production of polyhydroxybutyrate (PHB) in Azohydromonas lata bacteria, was developed and validated using a comprehensive series of experimental measurements. The model accounted for biomass growth, biopolymer accumulation, carbon and nitrogen sources utilization, oxygen mass transfer and uptake rates and average molecular weights of the accumulated PHB, produced under batch and fed-batch cultivation conditions. Model predictions were in excellent agreement with experimental measurements. The validated model was subsequently utilized to calculate optimal operating conditions and feeding policies for maximizing PHB productivity for desired PHB molecular properties. More specifically, two optimal fed-batch strategies were calculated and experimentally tested: (1) a nitrogen-limited fed-batch policy and (2) a nitrogen sufficient one. The calculated optimal operating policies resulted in a maximum PHB content (94% g/g) in the cultivated bacteria and a biopolymer productivity of 4.2 g/(l h), respectively. Moreover, it was demonstrated that different PHB grades with weight average molecular weights of up to 1513 kg/mol could be produced via the optimal selection of bioprocess operating conditions.









Similar content being viewed by others
Abbreviations
- AcAcCoA:
-
Acetoacetyl coenzyme A
- AcCoA:
-
Acetyl coenzyme A
- AL:
-
Azohydromonas lata cultivation medium
- AL*:
-
A. lata feeding medium for the discrete single-step policy
- AL**:
-
A. lata feeding medium for the continuous policy
- ATP:
-
Adenosine triphosphate
- CS:
-
Continuous feeding policy
- CoA-SH:
-
Coenzyme A
- C/N:
-
Carbon to nitrogen mass ratio
- DNS:
-
Dinitrosalicylic acid
- DSM:
-
German collection of microorganisms and cell cultures
- FPT:
-
Fixed pivot technique
- FTIR:
-
Fourier transform infrared spectroscopy
- GC:
-
Gas chromatography
- GPC:
-
Gel permeation chromatography
- MWD:
-
Molecular weight distribution
- NADH:
-
Nicotinamide adenine dinucleotide
- phaA :
-
β-Ketothiolase
- phaB :
-
NADH-depended acetoacetyl reductase
- phaC :
-
PHA synthase
- phaZ :
-
PHA depolymerase
- PHAs:
-
Polyhydroxyalkanoates
- PHB:
-
Polyhydroxybutyrate
- PID:
-
Proportional–integral–derivative controller
- RI:
-
Refractive index
- SS:
-
Single-step feeding policy
- UV/Vis:
-
Ultraviolet–visible spectroscopy
- 3-HB:
-
3-Hydroxybutyric acid, 3-hydroxybutyrate
- 3-HBCoA:
-
3-Hydroxybutyric coenzyme A
- δ(t − t i ):
-
Kronecker delta function
- DCW:
-
Dry cell weight (g/l)
- [D n ]:
-
“Dead” polymer chains concentration (mol/l)
- D.O.:
-
Dissolved oxygen concentration (% g/g)
- [E-OH]:
-
Depolymerase concentration (mol/l)
- [E-SH]:
-
Synthase dimer concentration (mol/l)
- [H2O]:
-
Water concentration (g/l)
- \(J_{{{\text{M}}^{\#} }} (t )\) :
-
Monomer production rate (mol/h)
- k d :
-
Polymer degradation kinetic constant (1/h)
- k H :
-
Henry’s law constant for oxygen (mol/(l atm))
- k i :
-
Monomer initiation kinetic constant (1/h)
- k L α :
-
Volumetric oxygen transfer coefficient (l/h)
- k m :
-
Specific growth saturation constant (g/g)
- k m1 :
-
Intermediate monomer initiation constant (1/h)
- k m2 :
-
Intermediate polymer propagation constant (l/(mol h))
- k p :
-
Polymer propagation kinetic constant (1/h)
- k t , k ′ t :
-
Chain transfer to water kinetic constants (1/h)
- k 1–5 :
-
Macroscopic model kinetic constants
- [M]:
-
Monomer concentration (mol/l)
- M n :
-
Number average molecular weight (g/mol)
- [MS#]:
-
Monomer-synthase complex (E-SH-M#) concentration (mol/l)
- M w :
-
Weight average molecular weight (g/mol)
- MWM :
-
Monomer unit (3-HB) molecular weight
- \({\text{MW}}_{{{\text{O}}_{ 2} }}\) :
-
Oxygen molecular weight (g/mol)
- [M#]:
-
Monomer coenzyme A (M-SCoA) concentration (mol/l)
- [N]:
-
Ammonium sulfate concentration (g/l)
- N s :
-
Number of samples
- O.D.:
-
Optical density @ 600 nm
- [O2]:
-
Oxygen concentration (g/l)
- PDI:
-
Polydispersity index
- [P]:
-
PHB concentration (g/l)
- [P n ]:
-
“Live” polymer chains (P n -ES) concentration (mol/l)
- \([{\text{P}}_{n}^{*} ]\) :
-
“Intermediate” polymer chains (P n -ES-M#) concentration (mol/l)
- Q :
-
Volumetric flow rate (l/h)
- R :
-
Ideal gas constant ((l atm)/(mol K))
- \(R_{{{\text{D}}_{n} }}\) :
-
Net formation rate of “dead” polymer chains (mol/(l h))
- \(R_{{{\text{M}}^{\#} }}\) :
-
Net consumption rate of monomer (mol/(l h))
- \(R_{{{\text{MS}}^{\#} }}\) :
-
Net formation rate of synthase-monomer complex (mol/(l h))
- R n :
-
Ammonium sulfate consumption rate (g/(l h))
- \(R_{{{\text{O}}_{ 2} , {\text{gl}}}}\) :
-
Oxygen mass transfer rate (g/(l h))
- \(R_{{{\text{O}}_{ 2} }}\) :
-
Oxygen mass cells uptake rate (g/(l h))
- R p :
-
PHB production rate (g/(l h))
- \(R_{{{\text{P}}_{n} }}\) :
-
Net formation rate of “live” polymer chains (mol/(l h))
- \(R_{{{\text{P}}_{n}^{ *} }}\) :
-
Net formation rate of “intermediate” polymer chains (mol/(l h))
- R s :
-
Sucrose consumption rate (g/(l h))
- R X :
-
Residual biomass growth rate (g/(l h))
- [S]:
-
Sucrose concentration (g/l)
- T :
-
Absolute temperature (K)
- V :
-
Volume (l)
- w P :
-
Intracellular PHB content (g/g)
- [Χ]:
-
Residual biomass concentration (g/l)
- Y NX :
-
Ammonium sulfate consumption coefficient for residual biomass synthesis/maintenance (g/g)
- \(\dot{Y}_{\text{NX}}\) :
-
Ammonium sulfate consumption rate yield coefficient for residual biomass synthesis/maintenance (g/(g h))
- Y P/S :
-
Accumulated polymer per consumed sucrose yield (g/g)
- Y SP :
-
Sucrose consumption coefficient for PHB accumulation (g/g)
- Y SX :
-
Sucrose consumption coefficient for residual biomass synthesis/maintenance (g/g)
- Y X/S :
-
Total produced biomass per consumed sucrose yield (g/g)
- \(\dot{Y}_{\text{SX}}\) :
-
Sucrose consumption rate yield coefficient for residual biomass synthesis/maintenance (g/(g h))
- z :
-
Oxygen compressibility factor
- μ s :
-
Specific growth rate (l/h)
- eq:
-
Equilibrium
- g:
-
Gas phase
- in:
-
Feeding stream
- l:
-
Liquid phase
- max:
-
Maximum value
- o:
-
Initial value
- out:
-
Sample or outflow stream
- P:
-
PHB
- T:
-
Total
- X:
-
Residual biomass
References
Mozejko-Ciesielska J, Kiewisz R (2016) Bacterial polyhydroxyalkanoates: still fabulous? Microbiol Res 192:271–282
Akaraonye E, Keshavarz T, Roy I (2010) Production of polyhydroxyalkanoates: the future green materials of choice. J Chem Technol Biotechnol 85:732–743
Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J App Microbiol 102:1437–1449
Zinn M, Witholt B, Egli T (2001) Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev 53:5–21
Jiang G, Hill DJ, Kowalczuk M, Johnston B, Adamus G, Irorere V, Radecka I (2016) Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int J Mol Sci 17:1–21
Khanna S, Srivastava AK (2005) Recent advances in microbial polyhydroxyalkanoates. Process Biochem 40:607–619
Urtuvia V, Villegas P, González M, Seeger M (2014) Bacterial production of the biodegradable plastics polyhydroxyalkanoates. Int J Biol Macromol 70:208–213
Philip S, Keshavarz T, Roy I (2007) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol 82:233–247
Chen G-Q (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446
Penloglou G, Chatzidoukas C, Kiparissides C (2012) Microbial production of polyhydroxybutyrate with tailor-made properties: an integrated modelling approach and experimental validation. Biotechnol Adv 30:329–337
Dias JML, Serafim LS, Lemos PC, Reis MA, Oliveira R (2005) Mathematical modelling of a mixed culture cultivation process for the production of polyhydroxybutyrate. Biotechnol Bioeng 92:209–222
Johnson K, Kleerebezem R, van Loosdrecht MCM (2010) Influence of the C/N ratio on the performance of polyhydroxybutyrate (PHB) producing sequencing batch reactors at short SRTs. Water Res 44:2141–2152
Somashekara DM, Rastogi NK, Ramachandriah T (2009) A simple kinetic model for growth and biosynthesis of polyhydroxyalkanoate in Bacillus flexus. New Biotechnol 26:92–98
Purushothaman M, Anderson RKI, Narayana S, Jayaraman VK (2001) Industrial byproducts as cheaper medium components influencing the production of polyhydroxyalkanoates (PHA)—biodegradable plastics. Bioprocess Biosyst Eng 24:131–136
Garcia-Torreiro M, Lu-Chau TA, Lema JM (2016) Effect of nitrogen and/or oxygen concentration on poly(3-hydroxybutyrate) accumulation by Halomonas boliviensis. Bioprocess Biosyst Eng 39:1365–1374
Basak B, Ince O, Artan N, Yagci N, Ince BK (2011) Effect of nitrogen limitation on enrichment of activated sludge for PHA production. Bioprocess Biosyst Eng 34:1007–1016
Kaur G, Roy I (2015) Strategies for large-scale production of polyhydroxyalkanoates. Chem Biochem Eng Q 29:157–172
Mozumder MSI, Goormachtigh L, Garcia-Gonzalez L, De Wever H, Volcke EIP (2014) Modeling pure culture heterotrophic production of polyhydroxybutyrate (PHB). Biores Technol 155:272–280
Spoljaric IV, Lopar M, Koller M, Muhr A, Salerno A, Reiterer A, Malli K, Angerer H, Strohmeier K, Schober S, Mittelbach M, Horvat P (2013) Mathematical modeling of poly[(R)-3-hydroxyalkanoate] synthesis by Cupriavidus necator DSM 545 on substrates stemming from biodiesel production. Biores Technol 133:482–494
Spoljaric IV, Lopar M, Koller M, Muhr A, Salerno A, Reiterer A, Horvat P (2013) In silico optimization and low structured kinetic model of poly[(R)-3-hydroxybutyrate] synthesis by Cupriavidus necator DSM545 by fed-batch cultivation on glycerol. J Biotechnol 168:625–635
Novak M, Koller M, Braunegg G, Horvat P (2015) Mathematical modelling as a tool for optimized PHA production. Chem Biochem Eng Q 29:183–220
Khanna S, Srivastava AK (2005) A simple structured mathematical model for biopolymer (PHB) production. Biotechnol Prog 21:830–838
Wang B, Sharma-Shivappa RR, Olson JW, Khan SA (2012) Upstream process optimization of polyhydroxybutyrate (PHB) by Alcaligenes latus using two-stage batch and fed-batch fermentation strategies. Bioprocess Biosyst Eng 35:1591–1602
Horvat P, Spoljaric IV, Lopar M, Atlic A, Koller M, Braunegg G (2013) Mathematical modelling and process optimization of a continuous 5-stage bioreactor cascade for production of poly[-(R)-3-hydroxybutyrate] by Cupriavidus necator. Bioprocess Biosyst Eng 36:1235–1250
Lopez-Arenas T, González-Contreras M, Anaya-Rezac O, Sales-Cruz M (2017) Analysis of the fermentation strategy and its impact on the economics of the production process of PHB (polyhydroxybutyrate). Com Chem Eng. doi:10.1016/j.compchemeng.2017.03.009 (in press)
Penloglou G, Roussos A, Chatzidoukas C, Kiparissides C (2010) A combined metabolic/polymerization kinetic model on the microbial production of poly(3-hydroxybutyrate). New Biotechnol 27:358–367
Chatzidoukas C, Penloglou G, Kiparissides C (2013) Development of a structured dynamic model for the production of polyhydroxybutyrate (PHB) in Azohydromonas lata cultures. Biochem Eng J 71:72–80
Penloglou G, Chatzidoukas C, Roussos A, Kiparissides C (2011) Model-based dynamic optimisation of microbial processes for the high-yield production of biopolymers with tailor-made molecular properties. Comput Aided Chem Eng 29:1401–1405
Penloglou G, Kretza E, Chatzidoukas C, Parouti S, Kiparissides C (2012) On the control of the molecular weight distribution of the microbially produced polyhydroxybutyrate by Alcaligenes latus. Biochem Eng J 62:39–47
Wang F, Lee SY (1997) Poly(3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl Environ Microb 63:3703–3706
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Naik S, Gopal SKV, Somal P (2008) Bioproduction of polyhydroxyalkanoates from bacteria: a metabolic approach. World J Microb Biotechnol 24:2307–2314
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Müh U, Sinskey AJ, Kirby DP, Lane WS, Stubbe JA (1999) PHA synthase from Chromatium vinosum: cysteine 149 is involved in covalent catalysis. Biochemistry 38:826–837
Rehm BHA, Steinbüchel A (1999) Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int J Biol Macromol 25:3–19
Zhang S, Yasuo T, Lenz RW, Goodwin S (2000) Kinetic and mechanistic characterization of the polyhydroxybutyrate synthase from Ralstonia eutropha. Biomacromol 1:244–251
Shrivastav A, Kim H-Y, Kim Y-R (2013) Advances in the applications of polyhydroxyalkanoate nanoparticles for novel drug delivery system. Biomed Res Int 581684:1–12
Kawaguchi Y (1992) Doi Y Kinetics and mechanism of synthesis and degradation of poly(3-hydroxybutyrate) in Alcaligenes eutrophus. Macromolecules 25:2324–2329
Haywood GW, Anderson AJ, Dawes EA (1989) The importance of PHB-synthase substrate specificity in polyhydroxyalkanoate synthesis by Alcaligenes eutrophus. FEMS Microbiol Lett 57:1–6
Dean JA (1998) Lange’s handbook of chemistry, 15th edn. McGraw-Hill, New York
Galaction AI, Cascaval D, Oniscu C, Turnea M (2004) Prediction of oxygen mass transfer coefficients in stirred bioreactors for bacteria, yeasts and fungus broths. Biochem Eng J 20:85–94
Tolosa L, Kostov Y, Harms P, Rao G (2002) Noninvasive measurement of dissolved oxygen in shake flasks. Biotechnol Bioeng 80:594–597
Ren Q, Roo G, Ruth K, Witholt B, Zinn M, Thony-Meyer L (2009) Simultaneous accumulation and degradation of polyhydroxyalkanoates: futile cycle or clever regulation? Biomacromol 10:916–922
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Penloglou, G., Vasileiadou, A., Chatzidoukas, C. et al. Model-based intensification of a fed-batch microbial process for the maximization of polyhydroxybutyrate (PHB) production rate. Bioprocess Biosyst Eng 40, 1247–1260 (2017). https://doi.org/10.1007/s00449-017-1784-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1784-0