Abstract
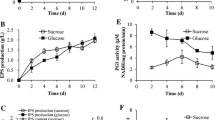
This study aimed to improve the production of polysaccharide by engineering the biosynthetic pathway in Ganoderma lucidum through the overexpression of α-phosphoglucomutase (PGM) gene. PGM is responsible for the linkage between sugar catabolism and sugar anabolism. The effects of PGM gene overexpression on intracellular polysaccharide (IPS) content, extracellular polysaccharide (EPS) production and transcription levels of three genes encoding the enzymes involved in polysaccharide biosynthesis, including PGM, UDP-glucose pyrophosphorylase (UGP), and β-1,3-glucan synthase (GLS), were investigated. The maximum IPS content and EPS production in G. lucidum overexpressing the PGM gene were 23.67 mg/100 mg dry weight and 1.76 g/L, respectively, which were higher by 40.5 and 44.3 % than those of the wild-type strain. The transcription levels of PGM, UGP and GLS were upregulated by 4.77-, 1.51- and 1.53-fold, respectively, in the engineered strain, suggesting that increased polysaccharide biosynthesis may result from a higher expression of those genes.




Similar content being viewed by others
References
Babitskaya VG, Shcherba VV, Puchkova TA, Smirnov DA (2005) Polysaccharides of Ganoderma lucidum: factors affecting their production. Appl Biochem Microbiol 41(2):169–173
Boels IC, Kleerebezem M, de Vos WM (2003) Engineering of carbon distribution between glycolysis and sugar nucleotide biosynthesis in Lactococcus lactis. Appl Environ Microbiol 69(2):1129–1135
Boels IC, Ramos A, Kleerebezem M, De Vos WM (2001) Functional analysis of the Lactococcus lactis galU and galE genes and their impact on sugar nucleotide and exopolysaccharide biosynthesis. Appl Environ Microbiol 67(7):3033–3040
Boels IC, van Kranenburg R, Kanning MW, Chong BF, de Vos WM, Kleerebezem M (2003) Increased exopolysaccharide production in Lactococcus lactis due to increased levels of expression of the NIZO B40 eps gene cluster. Appl Environ Microbiol 69(8):5029–5031
Chen H, Yan M, Zhu J, Xu X (2011) Enhancement of exo-polysaccharide production and antioxidant activity in submerged cultures of Inonotus obliquus by lignocellulose decomposition. J Ind Microbiol Biotechnol 38(2):291–298
Chen SL, Xu J, Liu C, Zhu YJ, Nelson DR, Zhou SG, Li CF, Wang LZ, Guo X, Sun YZ, Luo HM, Li Y, Song JY, Henrissat B, Levasseur A, Qian J, Li JQ, Luo X, Shi LC, He L, Xiang L, Xu XL, Niu YY, Li QS, Han MV, Yan HX, Zhang J, Chen HM, Lv AP, Wang Z, Liu MZ, Schwartz DC, Sun C (2012) Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat Commun 3:913
Degeest B, De Vuyst L (2000) Correlation of activities of the enzymes alpha-phosphoglucomutase, UDP-galactose4-epimerase, and UDP-glucose pyrophosphorylase with exopolysaccharide biosynthesis by Streptococcus thermophilus LY03. Appl Environ Microbiol 66(8):3519–3527
Fang QH, Tang YJ, Zhong JJ (2002) Significance of inoculation density control in production of polysaccharide and ganoderic acid by submerged culture of Ganoderma lucidum. Process Biochem 37(12):1375–1379
Fang QH, Zhong JJ (2002) Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites-ganoderic acid and polysaccharide. Biochem Eng J 10(1):61–65
Gao H, Gu WY (2007) Optimization of polysaccharide and ergosterol production from Agaricus brasiliensis by fermentation process. Biochem Eng J 33(3):202–210
Gastebois A, Clavaud C, Aimanianda V, Latge JP (2009) Aspergillus fumigatus: cell wall polysaccharides, their biosynthesis and organization. Future Microbiol 4(5):583–595
Hardy GG, Caimano MJ, Yother J (2000) Capsule biosynthesis and basic metabolism in Streptococcus pneumoniae are linked through the cellular phosphoglucomutase. J Bacteriol 182(7):1854–1863
Hsieh C, Tseng MH, Liu CJ (2006) Production of polysaccharides from Ganoderma lucidum (CCRC 36041) under limitations of nutrients. Enzyme Microb Technol 38(1–2):109–117
Huang HC, Chen CI, Hung CN, Liu YC (2009) Experimental analysis of the oil addition effect on mycelia and polysaccharide productions in Ganoderma lucidum submerged culture. Bioproc Biosyst Eng 32(2):217–224
Lee KM, Lee SY, Lee HY (1999) Bistage control of pH for improving exopolysaccharide production from mycelia of Ganoderma lucidum in an air-lift fermentor. J Biosci Bioeng 88(6):646–650
Lee WY, Park Y, Ahn JK, Ka KH, Park SY (2007) Factors influencing the production of endopolysaccharide and exopolysaccharide from Ganoderma applanatum. Enzyme Microb Technol 40(2):249–254
Levander F, Svensson M, Radstrom P (2002) Enhanced exopolysaccharide production by metabolic engineering of Streptococcus thermophilus. Appl Environ Microbiol 68(2):784–790
Liu GQ, Zhang KC (2007) Enhancement of polysaccharides production in Ganoderma lucidum by the addition of ethyl acetate extracts from Eupolyphaga sinensis and Catharsius molossus. Appl Microbiol Biotechnol 74(3):572–577
Liu YS, Wu JY (2012) Effects of Tween 80 and pH on mycelial pellets and exopolysaccharide production in liquid culture of a medicinal fungus. J Ind Microbiol Biotechnol 39(4):623–628
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Sa-Correia I, Fialho AM, Videira P, Moreira LM, Marques AR, Albano H (2002) Gellan gum biosynthesis in Sphingomonas paucimobilis ATCC 31461: genes, enzymes and exopolysaccharide production engineering. J Ind Microbiol Biotechnol 29(4):170–176
Spatafora G, Rohrer K, Barnard D, Michalek S (1995) A Streptococcus mutans mutant that synthesizes elevated levels of intracellular polysaccharide is hypercariogenic in vivo. Infect Immun 63(7):2556–2563
Tang YJ, Zhong JJ (2002) Exopolysaccharide biosynthesis and related enzyme activities of the medicinal fungus, Ganoderma lucidum, grown on lactose in a bioreactor. Biotechnol Lett 24(12):1023–1026
Tang YJ, Zhang W, Zhong JJ (2009) Performance analyses of a pH-shift and DOT-shift integrated fed-batch fermentation process for the production of ganoderic acid and Ganoderma polysaccharides by medicinal mushroom Ganoderma lucidum. Bioresour Technol 100(5):1852–1859
Thorne L, Mikolajczak MJ, Armentrout RW, Pollock TJ (2000) Increasing the yield and viscosity of exopolysaccharides secreted by Sphingomonas by augmentation of chromosomal genes with multiple copies of cloned biosynthetic genes. J Ind Microbiol Biotechnol 25(1):49–57
Torino MI, Mozzi F, Font de Valdez G (2005) Exopolysaccharide biosynthesis by Lactobacillus helveticus ATCC 15807. Appl Microbiol Biotechnol 68(2):259–265
Velasco SE, Yebra MJ, Monedero V, Ibarburu I, Duenas MT, Irastorza A (2007) Influence of the carbohydrate source beta-glucan production and enzyme activities involved in sugar metabolism in Pediococcus parvulus 2.6. Int J Food Microbiol 115(3):325–334
Wagner R, Mitchell DA, Sassaki GL, Amazonas M (2004) Links between morphology and physiology of Ganoderma lucidum in submerged culture for the production of exopolysaccharide. J Biotechnol 114(1–2):153–164
Wang SH, Liang CJ, Weng YW, Chen YH, Hsu HY, Chien HF, Tsai JS, Tseng YC, Li CY, Chen YL (2012) Ganoderma lucidum polysaccharides prevent platelet-derived growth factor-stimulated smooth muscle cell proliferation in vitro and neointimal hyperplasia in the endothelial-denuded artery in vivo. J Cell Physiol 227(8):3063–3071
Xu JW, Xu YN, Zhong JJ (2010) Production of individual ganoderic acids and expression of biosynthetic genes in liquid static and shaking cultures of Ganoderma lucidum. Appl Microbiol Biotechnol 85(4):941–948
Xu JW, Xu YN, Zhong JJ (2012) Enhancement of ganoderic acid accumulation by overexpression of an N-terminal truncated 3-hydroxy-3-methylglutaryl CoA reductase gene in the basidiomycete Ganoderma lucidum. Appl Environ Microbiol 78(22):7968–7976
Xu JW, Zhao W, Zhong JJ (2010) Biotechnological production and application of ganoderic acids. Appl Microbiol Biotechnol 87(2):457–466
Yu X, Ji SL, He YL, Reng MF, Xu JW (2014) Development of an expression plasmid and its use in genetic manipulation of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (higher basidiomycetes). Int J Med Mushrooms 16(2):161–168
Zhang BB, Cheung PCK (2011) Use of stimulatory agents to enhance the production of bioactive exopolysaccharide from Pleurotus tuber-regium by submerged fermentation. J Agric Food Chem 59(4):1210–1216
Zhang W, Tang YJ (2008) A novel three-stage light irradiation strategy in the submerged fermentation of medicinal mushroom Ganoderma lucidum for the efficient production of ganoderic acid and Ganoderma polysaccharides. Biotechnol Prog 24(6):1249–1261
Zhou JS, Ji SL, Ren MF, He YL, Jing XR, Xu JW (2014) Enhanced accumulation of individual ganoderic acids in a submerged culture of Ganoderma lucidum by the overexpression of squalene synthase gene. Biochem Eng J 90:178–183
Zhu LW, Tang YJ (2010) Significance of protein elicitor isolated from Tuber melanosporum on the production of ganoderic acid and Ganoderma polysaccharides during the fermentation of Ganoderma lucidum. Bioproc Biosyst Eng 33(8):999–1005
Zhu LW, Zhong JJ, Tang YJ (2008) Significance of fungal elicitors on the production of ganoderic acid and Ganoderma polysaccharides by the submerged culture of medicinal mushroom Ganoderma lucidum. Process Biochem 43(12):1359–1370
Zhu LW, Zhong JJ, Tang YJ (2010) Multi-fed batch culture integrated with three-stage light irradiation and multiple additions of copper ions for the hyperproduction of ganoderic acid and Ganoderma polysaccharides by the medicinal mushroom Ganoderma lucidum. Process Biochem 45(12):1904–1911
Acknowledgments
Financial support from the National Natural Science Foundation of China (31360495), the start-up grant from Kunming University of Science and Technology (KKSY201226107), Department of Science and Technology of Yunnan Province (2012BA015), CNTC (110201201009 BR-03) and Hongyun Honghe Tobacco (Group) Co. Ltd. (HYHH2012HX06) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
J.-W. Xu and S.-L. Ji have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, JW., Ji, SL., Li, HJ. et al. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene. Bioprocess Biosyst Eng 38, 399–405 (2015). https://doi.org/10.1007/s00449-014-1279-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1279-1