Abstract
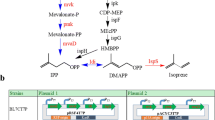
An engineered Escherichia coli strain was developed for enhanced isoprene production using d-galactose as substrate. Isoprene is a valuable compound that can be biosynthetically produced from pyruvate and glyceraldehyde-3-phosphate (G3P) through the methylerythritol phosphate pathway (MEP). The Leloir and De Ley–Doudoroff (DD) pathways are known existing routes in E. coli that can supply the MEP precursors from d-galactose. The DD pathway was selected as it is capable of supplying equimolar amounts of pyruvate and G3P simultaneously. To exclusively direct d-galactose toward the DD pathway, an E. coli ΔgalK strain with blocked Leloir pathway was used as the host. To obtain a fully functional DD pathway, a dehydrogenase encoding gene (gld) was recruited from Pseudomonas syringae to catalyze d-galactose conversion to d-galactonate. Overexpressions of endogenous genes known as MEP bottlenecks, and a heterologous gene, were conducted to enhance and enable isoprene production, respectively. Growth test confirmed a functional DD pathway concomitant with equimolar generation of pyruvate and G3P, in contrast to the wild-type strain where G3P was limiting. Finally, the engineered strain with combined DD–MEP pathway exhibited the highest isoprene production. This suggests that the equimolar pyruvate and G3P pools resulted in a more efficient carbon flux toward isoprene production. This strategy provides a new platform for developing improved isoprenoid producing strains through the combined DD–MEP pathway.




Similar content being viewed by others
References
Hong SY, Zurbriggen AS, Melis A (2012) Isoprene hydrocarbons production upon heterologous transformation of Saccharomyces cerevisiae. J Appl Microbiol 113:52–65
Weissermel K, Arpe HJ (2008) 1,3-Diolefins. Industrial organic chemistry. Wiley, Germany, pp 117–119
Lv X, Xu H, Yu H (2013) Significantly enhanced production of isoprene by ordered coexpression of genes dxs, dxr, and idi in Escherichia coli. Appl Microbiol Biotechnol 97:2357–2365
Lange BM, Rujan T, Martin W, Croteau R (2009) Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci USA 97:13172–13177
Kuzuyama T (2002) Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci Biotechnol Biochem 66:1619–1627
Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H (1996) Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc 8:2564–2566
Zhao Y, Yang J, Qin B, Li Y, Sun Y, Su S, Xian M (2011) Biosynthesis of isoprene in Escherichia coli via methylerythritol phosphate (MEP) pathway. Appl Microbiol Biotechnol 90:1915–1922
Ajikumar PK, Xiao W-H, Tyo KEJ, Wang Y, Simeon F, Leonard E et al (2010) Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science 330:70
Morrone D, Lowry L, Determan MK, Hershey DM, Xu M, Peters RJ (2010) Increasing diterpene yield with a modular metabolic engineering system in E. coli: comparison of MEV and MEP isoprenoid precursor pathway engineering. Appl Microbiol Biotechnol 85:1893–1906
Kim S-W, Keasling JD (2001) Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol Bioeng 72:408–415
Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21:796–802
Farmer WR, Liao JC (2001) Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol Prog 17:57–61
Liu H, Sun Y, Ramos KMR, Nisola GM, Valdehuesa KNG, Lee WK, Chung W-J (2013) Combination of Entner–Doudoroff pathway with MEP increases isoprene production in engineered Escherichia coli. PLoS One 8:e83290
Wi SG, Kim HJ, Mahadevan SA, Yang DJ, Bae HJ (2009) The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol 100:6658–6660
Malihan LB, Nisola GM, Mittal N, Seo JG, Chung WJ (2014) Blended ionic liquid systems for macroalgae pretreatment. Renew Energ 66:596–604
Holden HM, Rayment I, Thoden JB (2003) Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem 278:43885–43888
De Ley J, Doudoroff M (1957) The metabolism of d-galactose in Pseudomonas saccharophila. J Biol Chem 227:745–757
Cooper RA (1978) The utilization of d-galactonate and d-2-oxo-3-deoxygalactonate by Escherichia coli K-12. Arch Microbiol 118:199–206
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M et al (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:1–11
Sambrook J, Russell DW (2001) Molecular cloning-a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Novagen, λDE3 lysogenization kit user protocol TB031 Rev. C0805 2005 http://www.emdmillipore.com/life-science-research/technical-bulletins/c_IMOb.s1OXkUAAAEj2xsYzMkq. Accessed 7 Sept 2012
Liu H, Ramos KRM, Valdehuesa KNG, Nisola GM, Malihan LB, Lee WK, Chung W-J (2013) Metabolic engineering of Escherichia coli for biosynthesis of d-galactonate. Bioprocess Biosyst Eng. doi:10.1007/s00449-013-1003-6
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Esnouf MP, Harris RP, McVittie JD (1982) Triosephosphate isomerase from chicken and rabbit muscle. Methods Enzymol 89:579–583
Fleischer WR (1970) Enzymatic methods for lactic and pyruvic acids in Standard methods of clinical chemistry, vol 6. Academic Press USA, New York
Tomova N, Dimitrieva Setchenska M, Dimova O, Detchev G (1971) Spectrophotometric determination of the intermediates of glycolysis and the pentose phosphate cycle in Chlorella cells. Arch Microbiol 76:204–211
Lien OG (1959) Determination of gluconolactone, galactonolactone, and their free acids by hydroxamate method. Anal Chem 31:1363–1366
Yadav VG, De Mey M, Lim CG, Kumaran Ajikumar PK, Stephanopoulos G (2012) The future of metabolic engineering and synthetic biology: towards a systematic practice. Metab Eng 14:233–241
Zou R, Zhou K, Stephanopoulus G, Too HP (2013) Combinatorial engineering of 1-deoxy-d-xylulose 5-phosphate pathway using cross-lapping in vitro assembly (CLIVA) method. PLoS One 8:e79557
Chandran SS, Kealey JT, Reeves CD (2011) Microbial production of isoprenoids. Proc Biochem 46:1703–1710
Chang MCY, Keasling JD (2006) Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol 2:674–681
Sellick CA, Campbell RN, Reece RJ (2008) Galactose metabolism in yeast-structure and regulation of the Leloir pathway enzymes and the genes encoding them. Int Rev Cell Mol Biol 269:111–150
Flamholz A, Noor E, Bar-Even A, Liebermeister W, Milo R (2013) Glycolytic strategy as a tradeoff between energy yield and protein cost. Proc Natl Acad Sci USA 110:10039–10044
Peekhaus N, Conway T (1998) What’s for dinner?: Entner–Doudoroff metabolism in Escherichia coli. J Bacteriol 180:3495–3502
Szumilo T (1981) Pathway for d-galactonate catabolism in nonpathogenic mycobacteria. J Bacteriol 148:368–370
Arias A, Cerveñansky C (1986) Galactose metabolism in Rhizobium meliloti L5-30. J Bacteriol 167:1092–1094
Deacon J, Cooper RA (1977) d-Galactonate utilization by enteric bacteria. FEBS Lett 77:201–205
Wong TY, Yao X (1994) The De Ley–Doudoroff pathway of galactose metabolism in Azotobacter vinelandii. Appl Environ Microbiol 60:2065–2068
Hua SS, Markovitz A (1974) Multiple regulation of galactose operon—genetic evidence for a distinct site in the galactose operon that responds to capr gene regulation in Escherichia coli K-12. Proc Natl Acad Sci USA 71:507–511
Acknowledgments
This work was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2009-0093816).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramos, K.R.M., Valdehuesa, K.N.G., Liu, H. et al. Combining De Ley–Doudoroff and methylerythritol phosphate pathways for enhanced isoprene biosynthesis from d-galactose. Bioprocess Biosyst Eng 37, 2505–2513 (2014). https://doi.org/10.1007/s00449-014-1228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1228-z