Abstract
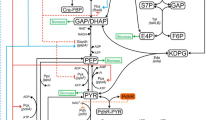
A kinetic-metabolic model approach describing and simulating Chinese hamster ovary (CHO) cell behavior is presented. The model includes glycolysis, pentose phosphate pathway, TCA cycle, respiratory chain, redox state and energetic metabolism. Growth kinetic is defined as a function of the major precursors for the synthesis of cell building blocks. Michaelis–Menten type kinetic is used for metabolic intermediates as well as for regulatory functions from energy shuttles (ATP/ADP) and cofactors (NAD/H and NADP/H). Model structure and parameters were first calibrated using results from bioreactor cultures of CHO cells expressing recombinant t-PA. It is shown that the model can simulate experimental data for all available experimental data, such as extracellular glucose, glutamine, lactate and ammonium concentration time profiles, as well as cell energetic state. A sensitivity analysis allowed identifying the most sensitive parameters. The model was then shown to be readily adaptable for studying the effect of sodium butyrate on CHO cells metabolism, where it was applied to the cases with sodium butyrate addition either at mid-exponential growth phase (48 h) or at the early plateau phase (74 h). In both cases, a global optimization routine was used for the simultaneous estimation of the most sensitive parameters, while the insensitive parameters were considered as constants. Finally, confidence intervals for the estimated parameters were calculated. Results presented here further substantiate our previous findings that butyrate treatment at mid-exponential phase may cause a shift in cellular metabolism toward a sustained and increased efficiency of glucose utilization channeled through the TCA cycle.










Similar content being viewed by others
References
Galbraith DJ, Tait AS, Racher AJ, Birch JR, James DC (2006) Control of culture environment for improved polyethylenimine-mediated transient production of recombinant monoclonal antibodies by CHO cells. Biotechnol Prog 22:753–762
Sunstrom NS, Gay RD, Wong DC, Kitchen NA, Deboer L, Gray PP (2000) Insulin-like growth factor-i and transferrin mediate growth and survival of Chinese hamster ovary cells. Biotechnol Prog 16:698–702
Fussenegger M, Schlatter S, Datwyler D, Mazur X, Bailey JE (1998) Controlled proliferation by multigene metabolic engineering enhances the productivity of Chinese hamster ovary cells. Nat Biotechnol 16:468–472
Cacciatore JJ, Chasin LA, Leonard EF (2010) Gene amplification and vector engineering to achieve rapid and high-level therapeutic protein production using the Dhfr-based CHO cell selection system. Biotechnol Adv 28:673–681
Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L et al (2003) Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol 21:414–421
Elliott S, Chang D, Delorme E, Dunn C, Egrie J, Giffin J, Lorenzini T, Talbot C, Hesterberg L (2004) Structural requirements for additional N-linked carbohydrate on recombinant human erythropoietin. J Biol Chem 279:16854–16862
Elliott S, Egrie J, Browne J, Lorenzini T, Busse L, Rogers N, Ponting I (2004) Control of rHuEPO biological activity: the role of carbohydrate. Exp Hematol 32:1146–1155
Quek LE, Dietmair S, Kromer JO, Nielsen LK (2010) Metabolic flux analysis in mammalian cell culture. Metab Eng 12:161–171
Kumar N, Gammell P, Clynes M (2007) Proliferation control strategies to improve productivity and survival during CHO based production culture. Cytotechnology 53:33–46
Birch JR, Racher AJ (2006) Antibody production. Adv Drug Deliv Rev 58:671–685
Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22:1393–1398
Jenkins N (2007) Modifications of therapeutic proteins: challenges and prospects. Cytotechnology 53:121–125
Chassagnole C, Noisommit-Rizzi N, Schmid JW, Mauch K, Reuss M (2002) Dynamic modeling of the central carbon metabolism of Escherichia coli. Biotechnol Bioeng 79:53–73
Nolan RP, Lee K (2011) Dynamic model of CHO cell metabolism. Metab Eng 13:108–124
Cloutier M, Perrier M, Jolicoeur M (2007) Dynamic flux cartography of hairy roots primary metabolism. Phytochemistry 68:2393–2404
Hendrick V, Winnepenninckx P, Abdelkafi CV, Andeputte O, Cherlet M, Marique T, Renemann G, Loa A, Kretzmer G, Werenne J (2001) Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology 36:71–83
Kim NS, Lee GM (2001) Overexpression of bcl-2 inhibits sodium butyrate-induced apoptosis in Chinese hamster ovary cells resulting in enhanced humanized antibody production. Biotechnol Bioeng 71:184–193
Lee SK, Lee GM (2003) Development of apoptosis resistant dihydrofolate reductase-deficient Chinese hamster ovary cell line. Biotechnol Bioeng 82:872–876
Palermo DP, DeGruf ME, Marotti KR, Rehberg E, Post LE (1992) Production of analytical quantities of recombinant proteins in Chinese hamster ovary cells using sodium butyrate to elevate gene expression. J Biotechnol 19:35–48
McMurray-Beaulieu V, Hisiger S, Durand C, Perrier M, Jolicoeur M (2009) Na-butyrate sustains energetic states of metabolism and t-PA productivity of CHO cells. J Biosci Bioeng 108:160–167
Jolicoeur M, Chavarie C, Carreau PJ, Archambault J (1992) Development of a helical-ribbon impeller bioreactor for high-density plant cell suspension culture. Biotechnol Bioeng 39:511–521
Ryll T, Wagner R (1991) Improved ion-pair high-performance liquid chromatographic method for the quantification of a wide variety of nucleotides and sugar nucleotides in animal cells. J Chromatogr 570:77–88
Leduc M, Tikhomiroff C, Cloutier M, Perrier M, Jolicoeur M (2006) Development of a kinetic metabolic model: application to Catharanthus roseus hairy root. Bioprocess Biosyst Eng 28:295–313
Zamoranoa F, Vande Wouwera A, Bastin G (2010) A detailed metabolic flux analysis of an underdetermined network of CHO cells. J Biotechnol 150:495–508
Martens DE (2007) Metabolic flux analysis of mammalian cells. Cell Eng 5:275–299
Ahn WC, Antoniewicz MR (2011) Metabolic flux analysis of CHO cells at growth and non-growth phases using isotopic tracers and mass spectrometry. Metab Eng 13:598–609
Bohnensack R (1981) Control of energy transformation of mitochondria: analysis by a quantitative model. Biochim Biophys Act 634:203–218
Garfinkel D (1971) Simulation of the Krebs cycle and closely related metabolism in perfused rat liver. I. Construction of a model. Comput Biomed Res 4:1–17
Garfinkel D (1971) Simulation of the Krebs cycle and closely related metabolism in perfused rat liver. II. Properties of the model. Comput Biomed Res 4:18–42
Korzeniewski B (1991) An extended dynamic model of oxidative phosphorylation. Biochim Biophys Acta 1060:210–223
Wu F, Yang F, Vinnakota KC, Beard DA (2007) Computer modeling of mitochondrial tricarboxylic acid cycle, oxidative phosphorylation, metabolite transport, and electrophysiology. J Biol Chem 282:24525–24537
Kauffman LG, Pajerowski JD, Jamshidi N, Palsson BO, Edwards JS (2002) Description and analysis of metabolic connectivity and dynamics in the human red blood cell. Biophys J 83:646–662
Dash RK, DiBella JA, Cabrera ME (2007) A computational model of skeletal muscle metabolism linking cellular adaptations induced by altered loading states to metabolic responses during exercise. Biomol Eng 6:14
Cloutier M, Bouchard-Marchand E, Perrier M, Jolicoeur M (2008) A predictive nutritional model for plant cells and hairy roots. Biotechnol Bioeng 99:189–200
Lambeth MJ, Kushmerick MJ (2002) A computational model for glycogenolysis in skeletal muscle. Ann Biomed Eng 30:808–827
Goudar C, Biener R, Boisart C, Heidemann R, Piret J, deGraaf A, Konstantinov K (2010) Metabolic flux analysis of CHO cells in perfusion culture by metabolite balancing and 2D[13C, 1H] COSYNMR spectroscopy. Metab Eng 12:138–149
Holzhutter HG (2004) The principle of flux minimization and its application to estimate stationary fluxes in metabolic networks. Eur J Biochem 271:2905–2922
Li JH, Sun XM, Zhang YX (2006) Improvement of hepatitis B surface antigen expression by dimethyl sulfoxide in the culture of recombinant Chinese hamster ovary cells. Process Biochem 41:317–322
Lu S, Sun X, Shi H, Zhang Y (2003) Determination of tricarboxylic acid cycle acids and other related substances in cultured mammalian cells by gradient ion-exchange chromatography with suppressed conductivity detection. J Chromatogr A 1012:161–168
Zhang F, Sun W, Yi X, Zhang Y (2006) Metabolic characteristics of recombinant Chinese hamster ovary cells expressing glutamine synthetase in presence and absence of glutamine. Cytotechnology 51:21–28
Sellick CA, Hansen R, Maqsood AR, Dunn WB, Stephens GM, Goodacre R et al (2009) Effective quenching processes for physiologically valid metabolite profiling of suspension cultured mammalian cells. Anal Chem 81:174–183
Maier K, Hofmann U, Reuss M, Mauch K (2007) Identification of metabolic fluxes in hepatic cells from transient 13C-labeling experiments: part II. Flux estimation. Biotechnol Bioeng 100:344–354
Ritter JB, Wahl AS, Freund S, Genzel Y, Reichl U (2010) Metabolic effects of influenza virus infection in cultured animal cells: intra- and extracellular metabolite profiling. BMC Syst Biol 4:61
Lu S, Sun X, Zhang Y (2005) Insight into metabolism of CHO cells at low glucose concentration on the basis of the determination of intracellular metabolites. Process Biochem 40:1917–1921
Kontoravdi C, Wong D, Lam C, Lee Y, Yap M, Pistikopoulos E, Mantalaris A (2007) Modeling amino acid metabolism in mammalian cells—towards the development of a model library. Biotechnol Prog 23:1261–1269
De Leon GM, Wlaschin KF, Nissom PM, Yap M, Hu WS (2007) Comparative transcriptional analysis of mouse hybridoma and recombinant Chinese hamster ovary cells undergoing butyrate treatment. J Biosci Bioeng 103:82–91
Yee JC, De Leon GM, Philp RJ, Yap M, Hu WS (2008) Genomic and proteome exploration of CHO and hybridoma cells under sodium butyrate treatment. Biotechnol Bioeng 99:1186–1204
Bode BP, Souba WW (1994) Modulation of cellular proliferation alters glutamine transport and metabolism in human hepatoma cells. Ann Surg 220:411–424
Rizhaupt A, Ellis A, Hosie KB, P Shirazi-Beechey S (1998) The characterization of butyrate transport across pig and human colonic luminal membrane. J Physiol 507:819–830
Beauvieux MC, Tissier P, Gin H, Canioni P, Gallis JL (1986) Butyrate impairs energy metabolism in isolated perfused liver of fed rats. J Nutr 131:1986–1992
Wlaschin KF, Hu WS (2006) Fedbatch culture and dynamic nutrient feeding. Adv Biochem Eng Biotechnol 101:43–74
Follstad BD, Balcarel RR, Stephanopoulos G, Wang D (1999) Metabolic flux analysis of hybridoma continuous culture steady state multiplicity. Biotechnol Bioeng 63:675–683
Bonarius HP, Hatzimanikatis V, Meesters KP, de Gooijer CD, Schmid G, Tramper J (1996) Metabolic flux analysis of hybridoma cells in different culture media using mass balances. Biotechnol Bioeng 50:299–318
Maier K, Hofmann U, Reuss M, Mauch K (2008) Identification of metabolic fluxes in hepatic cells from transient 13C-labeling experiments: part II. Flux estimation. Biotechnol Bioeng 100:355–370
Altamirano C, Illanes A, Casablancas A, Gámez X, Cairó JJ, Gòdia C (2001) Analysis of CHO cells metabolic redistribution in a glutamate-based defined medium in continuous culture. Biotechnol Prog 17:1032–1041
Van der Valk P, Gille JJP, van der Plas LHW, Jongkind JF, Verkerk A, Konings AWT, Joenje H (1988) Characterization of oxygen-tolerant Chinese hamster ovary cells: II. Energy metabolism and antioxidant status 4:345–356
Acknowledgments
This project was funded by the MabNet Research Network of the Natural Sciences and Engineering Research Council of Canada (NSERC) (MJ) and by an NSERC Discovery grant to MJ and OH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
449_2012_804_MOESM2_ESM.tif
Supplementary Fig. 1 Simulated data of intracellular metabolites for Control (solid line), and cultures with sodium butyrate addition at 48 h (NaBu-48h) (dashed line) and at 74 h (NaBu-74h) (dotted line). Axis units are mmol (106 cells)−1. Simulated values of intracellular concentrations showed to be within the range found in literature for TCA cycle intermediates (10−7–10−6 mmol (106 cells)−1) [39, 40], intracellular sugar phosphates (10−7–10−6 mmol (106 cells)−1) (CHO cells: [41]; Hepatic cells: [42]; MDCK cells: [43]), intracellular glutamine (10−7–10−6 mmol (106 cells)−1) and glutamate (10−6–10−5 mmol (106 cells)−1) (CHO cells: [44, 45]; HEK-293 cells: [45]). (TIFF 560 kb)
Rights and permissions
About this article
Cite this article
Ghorbaniaghdam, A., Henry, O. & Jolicoeur, M. A kinetic-metabolic model based on cell energetic state: study of CHO cell behavior under Na-butyrate stimulation. Bioprocess Biosyst Eng 36, 469–487 (2013). https://doi.org/10.1007/s00449-012-0804-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0804-3