Abstract
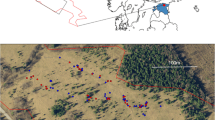
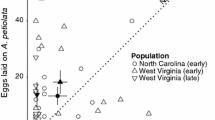
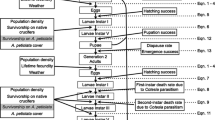
Non-native plants may be unpalatable or toxic, but have oviposition cues similar to native plants used by insects. The herbivore will then oviposit on the plant, but the offspring will be unable to develop. While such instances have been described previously, the fitness costs at the population level in the wild due to the presence of the lethal host have not been quantified, for this or other related systems. We quantified the fitness cost in the field for the native butterfly Pieris macdunnoughii in the presence of the non-native crucifer Thlaspi arvense, based on the spatial distributions of host plants, female butterflies and eggs in the habitat and the survival of the larvae in the wild. We found that 2.9 % of eggs were laid on T. arvense on average, with a survival probability of 0, yielding a calculated fitness cost of 3.0 % (95 % confidence interval 1.7–3.6 %) due to the presence of the non-native in the plant community. Survival probability to the pre-pupal stage for eggs laid on two native crucifers averaged 1.6 % over 2 years. The magnitude of the fitness cost will vary temporally and spatially as a function of the relative abundance of the non-native plant. We propose that the fine-scale spatial structure of the plant community relative to the butterflies’ dispersal ability, combined with the females’ broad habitat use, contributes to the fitness costs associated with the non-native plant and the resulting evolutionary trap.







Similar content being viewed by others
References
Bergman KO (1999) Habitat utilization by Lopinga achine (Nymphalidae: Satyrinae) larvae and ovipositing females: implications for conservation. Biol Conserv 88:69–74
Boggs CL, Wiklund C (2012) When putative host plants invade: perfomance and heritability of performance of butterfly larvae in the native and non-native plant range (in preparation)
Boggs CL et al (2012) Introduced host and native herbivore: opportunities for evolution in the face of maladaptation (in preparation)
Bowden SR (1971) American white butterflies (Pieridae) and English food-plants. J Lepidopterists Soc 25:6–12
Carlsson NOL, Sarnelle O, Strayer DL (2009) Native predators and exotic prey—an acquired taste? Front Ecol Environ 7:525–532
Carroll SP (2007) Natives adapting to invasive species: ecology, genes and the sustainability of conservation. Ecol Res 22:892–901
Casagrande RA, Dacey JE (2007) Monarch butterfly oviposition on swallow-worts (Vincetoxicum spp.). Environ Entomol 36:631–636
Chew FS (1974) Strategies of food plant exploitation in a complex of oligophagous butterflies (Lepidoptera). PhD dissertation, Biology. Yale University, New Haven
Chew FS (1975) Coevolution of Pierid butterflies and their cruciferous foodplants 1. The relative quality of available resources. Oecologia 20:117–127
Chew FS (1977) Coevolution of Pierid butterflies and their cruciferous foodplants 2. Distribution of eggs on potential foodplants. Evolution 31:568–579
Chew FS, Watt WB (2006) The green-veined white (Pieris napi L.), its Pierine relatives, and the systematics dilemmas of divergent character sets (Lepidoptera, Pieridae). Biol J Linn Soc 88:413–435
Courant AV, Holbrook AE, Van der Reijden ED, Chew FS (1994) Native pierine butterfly (Pieridae) adapting to naturalized crucifer? J Lepidopterists Soc 48:168–170
Cushman JH, Boggs CL, Weiss SB, Murphy DD, Harvey AW, Ehrlich PR (1994) Estimating female reproductive success of a threahened butterfly: influence of emergence time and hostplant phenology. Oecologia 99:194–200
Doak P, Kareiva P, Kingsolver J (2006) Fitness consequences of choosy oviposition for a time-limited butterfly. Ecology 87:395–408
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in colevolution. Evolution 18:586–608
Feldman TS, Haber WA (1998) Oviposition behavior, host plant use, and diet breadth of Anthanassa butterflies (Lepidoptera: Nymphalidae) using plants in the Acanthaceae in a Costa Rican community. Fl Entomol 81:396–406
Graves SD, Shapiro AM (2003) Exotics as host plants of the California butterfly fauna. Biol Conserv 110:413–433
Harvey JA, Bukovinszky T, van der Putten WH (2010) Interactions between invasive plants and insect herbivores: a plea for a multitrophic perspective. Biol Conserv 143:2251–2259
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18
Janz N, Bergstrom A, Johansson J (2005) Frequency dependence of host plant choice within and between patches: a large cage experiment. Evol Ecol 19:289–302
Keeler MS, Chew FS (2008) Escaping an evolutionary trap: preference and performance of a native insect on an exotic invasive host. Oecologia 156:559–568
Kuussaari M, van Houhuys S, Hellmann JJ, Singer MC (2004) Larval biology of checkerspots. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a model system for population biology. Oxford University Press, Oxford, pp 138–160
Lockwood JL, Hoopes M, Marchetti M (2007) Invasion ecology. Blackwell, Malden
Logarzo GA, Casalinuovo MA, Piccinali RV, Braun K, Hasson E (2011) Geographic host use variability and host range evolutionary dynamics in the phytophagous insect Apagomerella versicolor (Cerambycidae). Oecologia 165:387–402
McNeely JA (2006) As the world gets smaller, the chances of invasion grow. Euphytica 148:5–15
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci USA 98:5446–5451
Phillips BL, Shine R (2004) Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proc Natl Acad Sci USA 101:17150–17155
Pyke DA, Thompson JN (1986) Statistical analysis of survival and removal rate experiments. Ecology 67:240–245
R Development Core Team (2009) A language and environment for statistical computing, 2.3.0 edn. R Foundation for Statistical Computing, Vienna
Rodman JE, Chew FS (1980) Phytochemical correlates of herbivory in a community of native and naturalized Cruciferae. Biochem Syst Ecol 8:43–50
Schlaepfer MA, Sherman PW, Blossey B, Runge MC (2005) Introduced species as evolutionary traps. Ecol Lett 8:241–246
Sei M, Porter AH (2003) Microhabitat-specific early-larval survival of the maritime ringlet (Coenonympha tullia nipisiquit). Anim Conserv 6:55–61
Straatman R (1962) Note on certain Lepidoptera ovipositing on plants which are toxic to their larvae. J Lepidopterists Soc 16:99–103
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:357–374
Thompson JN (1994) The coevolutionary process. University of Chicago Press, Chicago
Warwick SI, Francis A, Susko DJ (2002) The biology of Canadian weeds 9. Thlaspi arvense L. (updated). Can J Plant Sci 82:803–823
Western Region Climate Center (2011) Monthly temperature listings. N Crested Butte, Colorado
Wheat CW, Vogel H, Wittstock U, Braby MF, Underwood D, Mitchell-Olds T (2007) The genetic basis of a plant-insect coevolutionary key innovation. Proc Natl Acad Sci USA 104:20427–20431
Acknowledgments
This work conforms to the legal requirements of the USA. The authors thank J. Bowsher, A. Cathcart, E. Forwand, and R. Neill for field assistance, and T. Fukami, R. McCoy, W. Watt, A. Porter and an anonymous reviewer for comments on the manuscript. Funding came from the Stanford Vice Provost for Undergraduate Education’s Biology Field Studies Program, NSF REU site grant DBI 9987953 to RMBL, and the Stanford University Center for Conservation Biology’s Bing Fund.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Konrad Fiedler.
Rights and permissions
About this article
Cite this article
Nakajima, M., Boggs, C.L., Bailey, S. et al. Fitness costs of butterfly oviposition on a lethal non-native plant in a mixed native and non-native plant community. Oecologia 172, 823–832 (2013). https://doi.org/10.1007/s00442-012-2537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2537-z