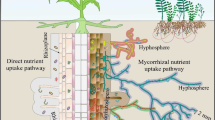
Abstract
Mosses play an integral role in the hydrologic regimes of ecosystems where they cover the soil surface, and thus affect biogeochemical cycling of elements influenced by soil oxidation–reduction (redox) reactions, including the plant growth-limiting nutrients, nitrogen and phosphorus (P). In rich fens where P often limits plant growth, we hypothesized that feedbacks between mosses and redox conditions would determine P availability to shallow-rooted forb species that constitute much of these wetlands’ unusually high plant species diversity. In a moss removal experiment in three fens, forb tissue P and microbial P were greater while anion exchange membrane (AEM) resin P was lower where mosses occurred than where they were removed, suggesting both higher availability and greater demand for P in moss-covered soils. Coupled physicochemical and biological mechanisms drove moss effects on P cycling, ultimately through effects on soil oxygenation or reduction: higher redox potential underlying mosses corresponded to greater microbial activity, phosphatase enzyme activity, and colonization by arbuscular mycorrhizal fungi (AMF), all of which can promote greater P availability to plants. These more oxidized soils stimulated: (1) greater microbial activity and root vigor; (2) correspondingly greater P demand via microbial uptake, forb uptake, and iron (Fe)-P reactions; and (3) greater P supply through soil and root phosphatase activity and AMF colonization. This work demonstrates that mosses improve vascular plant P acquisition by alleviating stresses caused by reducing conditions that would otherwise prevail in shallow underlying soils, thus providing a mechanism by which mosses facilitate plant species diversity in rich fens.





Similar content being viewed by others
References
Allen SE (1989) Analysis of vegetation and other organic materials. In: Allen SE (ed) Chemical analysis of ecological materials. Blackwell, Oxford, pp 46–61
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Bailey KM (2006) Effects of groundwater flow on soil chemistry, nutrient availability and plant species distributions in four New York State fens. PhD dissertation, Cornell University, Ithaca
Bates JW (2000) Mineral nutrition, substratum ecology, and pollution. In: Shaw AJ, Goffinet B (eds) Bryophyte biology. Cambridge University Press, Cambridge, pp 248–311
Bates JW, Bakken S (1998) Nutrient retention, desiccation and redistribution in mosses. In: Bates JW, Ashton NW, Duckett JG (eds) Bryology for the twenty-first century. British Bryological Society, Perth, pp 293–304
Bedford BL, Godwin KS (2003) Fens of the United States: distribution, characteristics, and scientific connection versus legal isolation. Wetlands 23:608–629
Bedford BL, Walbridge MR, Aldous A (1999) Patterns in nutrient availability and plant diversity of temperate North American wetlands. Ecology 80:2151–2169
Beringer J, Lynch AH, Chapin FS, Mack M, Bonan GB (2001) The representation of arctic soils in the land surface model: the importance of mosses. J Clim 14:3324–3335
Boomer KMB, Bedford BL (2008a) Groundwater-induced redox-gradients control soil properties and phosphorus availability across four headwater wetlands, New York, USA. Biogeochemistry 90:259–274
Boomer KMB, Bedford BL (2008b) Influence of nested groundwater systems on reduction-oxidation and alkalinity gradients with implications for plant nutrient availability in four New York fens. J Hydrol 351:107–125
Boyer MLH, Wheeler BD (1989) Vegetation patterns in spring-fed calcareous fens—calcite precipitation and constraints on fertility. J Ecol 77:597–609
Brady NC, Weil RR (2002) The nature and properties of soils, 13th edn. Prentice Hall, Upper Saddle River
Bret-Harte MS, Garcia EA, Sacre VM, Whorley JR, Wagner JL, Lippert SC, Chapin FS (2004) Plant and soil responses to neighbour removal and fertilization in Alaskan tussock tundra. J Ecol 92:635–647
Bret-Harte MS, Mack MC, Goldsmith GR, Sloan DB, DeMarco J, Shaver GR, Ray PM, Biesinger Z, Chapin FS (2008) Plant functional types do not predict biomass responses to removal and fertilization in Alaskan tussock tundra. J Ecol 96:713–726
Cheesman AW, Turner BL, Reddy KR (2010) Interaction of phosphorus compounds with anion-exchange membranes: implications for soil analysis. Soil Sci Soc Am J 74:1607–1612
Cornelissen JHC, Lang SI, Soudzilovskaia NA, During HJ (2007) Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Ann Bot 99:987–1001
Craft CB (2001) Biology of wetland soils. In: Richardson JL, Vepraskas MJ (eds) Wetland soils: genesis, hydrology, landscapes, and classification. Lewis, Boca Raton, pp 105–135
Crowley KF (2009) Mosses influence vascular plants in rich fens through effects on the biogeochemistry of shallow soils. PhD dissertation, Cornell University, Ithaca, NY
Glime JM, Wetzel RG, Kennedy BJ (1982) The effects of bryophytes on succession from alkaline marsh to Sphagnum bog. Am Midl Nat 108:209–223
Gornall JL, Jonsdottir IS, Woodin SJ, Van der Wal R (2007) Arctic mosses govern below-ground environment and ecosystem processes. Oecologia 153:931–941
Gutierrez JL, Jones CG (2006) Physical ecosystem engineers as agents of biogeochemical heterogeneity. Bioscience 56:227–236
Heijmans M, Arp WJ, Berendse F (2001) Effects of elevated CO2 and vascular plants on evapotranspiration in bog vegetation. Glob Change Biol 7:817–827
Heijmans M, Arp WJ, Chapin FS (2004) Controls on moss evaporation in a boreal black spruce forest. Global Biogeochem Cycles 18:1524–1530
Jackson MB, Ricard B (2003) Physiology, biochemistry, and molecular biology of plant root systems subjected to flooding of the soil. In: de Kroon H, Visser EJW (eds) Root ecology. Ecological studies, Vol. 168. Springer, Berlin, pp 193–213
Kuhn NL, Mendelssohn IA, McKee KL, Lorenzen B, Brix H, Miao SL (2002) Root phosphatase activity in Cladium jamaicense and Typha domingensis grown in Everglades soil at ambient and elevated phosphorus levels. Wetlands 22:794–800
Lajtha K, Driscoll CT, Jarrell WM, Elliot ET (1999) Soil P: characterization and total element analysis. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 115–142
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberber O (2006) SAS for mixed models, 2nd edn. SAS Cary
Loeffler UCM, Cypionka H, Loeffler J (2008) Soil microbial activity along an arctic-alpine altitudinal gradient from a seasonal perspective. Eur J Soil Sci 59:842–854
Longton RE (1992) The role of bryophytes and lichens in terrestrial systems. In: Bates JW, Farmer AM (eds) Bryophytes and lichens in a changing environment. Oxford University Press, New York, pp 32–76
Lovley DR, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Nilsson MC, Wardle DA (2005) Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front Ecol Environ 3:421–428
Proctor MCF (2000) Physiological ecology. In: Shaw AJ, Goffinet B (eds) Bryophyte biology. Cambridge University Press, Cambridge, pp 237–268
Qian P, Schoenau JJ (2002) Practical applications of ion exchange resins in agricultural and environmental soil research. Can J Soil Sci 82:9–21
Rejmankova E, Sirova D (2007) Wetland macrophyte decomposition under different nutrient conditions: relationships between decomposition rate, enzyme activities and microbial biomass. Soil Biol Biochem 39:526–538
Rice SK, Collins D, Anderson AM (2001) Functional significance of variation in bryophyte canopy structure. Am J Bot 88:1568–1576
Rochefort L (2000) New frontiers in bryology and lichenology—Sphagnum—a keystone genus in habitat restoration. Bryologist 103:503–508
Schat H (1984) A comparative ecophysiological study on the effects of waterlogging and submergence on dune slack plants—growth, survival and mineral nutrition in sand culture experiments. Oecologia 62:279–286
Sedia EG, Ehrenfeld JG (2003) Lichens and mosses promote alternate stable plant communities in the New Jersey Pinelands. Oikos 100:447–458
Sedia EG, Ehrenfeld JG (2005) Differential effects of lichens, mosses and grasses on respiration and nitrogen mineralization in soils of the New Jersey Pinelands. Oecologia 144:137–147
Sedia EG, Ehrenfeld JG (2006) Differential effects of lichens and mosses on soil enzyme activity and litter decomposition. Biol Fertil Soils 43:177–189
Sime AD, Abbott LL, Abbott SP (2002) A mounting medium for use in indoor air quality spore-trap analyses. Mycologia 94:1087–1088
Smolders AJP, Lamers LPM, Lucassen E, Van der Velde G, Roelofs JGM (2006) Internal eutrophication: how it works and what to do about it—a review. Chem Ecol 22:93–111
Snowden RED, Wheeler BD (1993) Iron toxicity to fen plant species. J Ecol 81:35–46
Stookey LL (1970) Ferrozine—a new spectrophotometric reagent for iron. Anal Chem 42:779
Sveinbjornsson B, Oechel WC (1992) Controls on growth and productivity of bryophytes: environmental limitations under current and anticipated conditions. In: Bates JW, Farmer AM (eds) Bryophytes and lichens in a changing environment. Oxford University Press, Oxford, pp 77–102
Tabatabai MA (1982) Soil enzymes. In: Methods of soil analysis Part 2: chemical and microbiological properties, 2nd edn. Soil Science Society of America, Madison, WI, pp 903–948
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. Bryologist 106:395–409
Turner BL, Baxter R, Ellwood NTW, Whitton BA (2001) Characterization of the phosphatase activities of mosses in relation to their environment. Plant Cell Envir 24:1165–1176
USDA and NRCS (2009) The PLANTS Database (http://plants.usda.gov, 28 April 2009). National Plant Data Center, Baton Rouge, LA
van Breemen N (1995) How Sphagnum bogs down other plants. Trends Ecol Evol 10:270–275
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Weishampel PA, Bedford BL (2006) Wetland dicots and monocots differ in colonization by arbuscular mycorrhizal fungi and dark septate endophytes. Mycorrhiza 16:495–502
West AW, Sparling GP (1986) Modifications to the substrate-induced respiration method to permit measurement of microbial biomass in soils of differing water contents. J Microbiol Meth 5:177–189
Wolfe BE, Weishampel PA, Klironomos JN (2006) Arbuscular mycorrhizal fungi and water table affect wetland plant community composition. J Ecol 94:905–914
Wright AL, Reddy KR (2001) Phosphorus loading effects on extracellular enzyme activity in everglades wetland soils. Soil Sci Soc Am J 65:588–595
Acknowledgments
Particular thanks to Alexander Cheesman for sharing his microbial P data for use in this study, and to Ramesh Reddy for facilitating this collaboration. Much appreciation to numerous individuals for help in the field and in the laboratory. Thanks also to Tim Fahey, Steve Hamilton, and Jed Sparks for insightful advice and comments. Funding for this research was provided by the Cornell Program in Biogeochemistry and Environmental Biocomplexity, a Hatch grant from the US Department of Agriculture, and the US Environmental Protection Agency (EPA) Science to Achieve Results (STAR) Graduate Fellowship Program. EPA has not officially endorsed this publication, and the views expressed herein may not reflect the views of the EPA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Håkan Wallander.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crowley, K.F., Bedford, B.L. Mosses influence phosphorus cycling in rich fens by driving redox conditions in shallow soils. Oecologia 167, 253–264 (2011). https://doi.org/10.1007/s00442-011-1970-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-1970-8