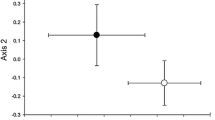
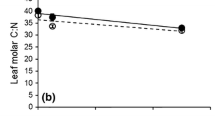
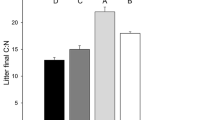
Abstract
In aquatic food webs consumers can affect other members of the web by releasing nutrients as a result of their feeding activity. There is increasing evidence of these positive effects on primary producers, but such nutrient regeneration can also affect detritivores, by favoring the activities of detritus-associated microbes. We examined the effects of nutrient regeneration by tadpoles on leaf-eating detritivores under laboratory conditions. We fed four species of tadpoles three different food items (leaf litter, algae, and sludgeworms). We then conditioned terrestrial dead leaves with water from reared tadpoles (treatments) or food items alone (controls), and compared the C:N ratios of the conditioned leaves and the growth of the isopod Asellus hilgendorfii fed on the conditioned leaves. Tadpole feeding activity reduced the C:N ratio of conditioned leaves, and the effect was greatest when tadpoles were fed algae. Isopod growth rates were often higher when they were fed the litter conditioned with water from reared tadpoles. Thus, nutrient regeneration by tadpoles had a positive indirect effect on detritivores by enhancing leaf quality. Tadpoles often occur in nutrient-limited habitats where leaf litter is the major energy source, and their facilitative effects on leaf-eating detritivores may be of great significance in food webs by enhancing litter decomposition.





Similar content being viewed by others
References
Alford RA (1999) Ecology: resource use, competition, and predation. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, pp 240–278
Bärlocher F, Kendrick B (1975) Leaf-conditioning by microorganisms. Oecologia 20:359–362
Biesterfeldt JM, Petranka JW, Sherbondy S (1993) Prevalence of chemical interference competition in natural populations of wood frogs, Rana sylvatica. Copeia 1993(3):688–695
Bowen SH (1987) Composition and nutritional value of detritus. In: Moriarty DJW, Pullin RSV (eds) Detritus and microbial ecology in aquaculture. International Center for Living Aquatic Resources Management, Manila, pp 192–216
Brönmark C, Rundle SD, Erlandsson A (1991) Interactions between freshwater snails and tadpoles: competition and facilitation. Oecologia 87:8–18
Cummins KW, Klug MJ (1979) Feeding ecology of stream invertebrates. Annu Rev Ecol Syst 10:147–172
Dickman M (1968) The effect of grazing by tadpoles on the structure of a periphyton community. Ecology 49:1188–1190
Elser JJ, Stabler LB, Hassett RP (1995) Nutrient limitation of bacterial growth and rates of bacterivory in lakes and oceans: a comparative study. Aquat Microb Ecol 9:105–110
Figueredo CC, Giani A (2005) Ecological interactions between Nile tilapia (Oreochromis niloticus, L.) and the phytoplanktonic community of the Furnas Reservoir (Brazil). Freshw Biol 50:1391–1403
Flecker AS, Feifarek BP, Taylor BW (1999) Ecosystem engineering by a tropical tadpole: density-dependent effects on habitat structure and larval growth rates. Copeia 1999(2):495–500
Geddes P, Trexler JC (2003) Uncoupling of omnivore-mediated positive and negative effects on periphyton mats. Oecologia 136:585–595
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Granéli W, Bertilsson S, Philibert A (2004) Phosphorus limitation of bacterial growth in high Arctic lakes and ponds. Aquat Sci 66:430–439
Haglund A, Hillebrand H (2005) The effect of grazing and nutrient supply on periphyton associated bacteria. FEMS Microbiol Ecol 52:31–41
Hatcher A (1991) Effect of temperature on carbon, nitrogen and phosphorus turnover by the solitary ascidian Herdmania momus (Savigny). J Exp Mar Biol Ecol 152:15–31
Hoff K, Blaustein AR, McDiarmid RW, Altig R (1999) Behavior: interactions and their consequences. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, pp 215–239
Holomuzki JR (1998) Grazing effects by green frog tadpoles (Rana clamitans) in a woodland pond. J Freshw Ecol 13:1–8
Irons JG, Oswood MW, Bryant PJ (1988) Consumption of leaf detritus by a stream shredder: influence of tree species and nutrient status. Hydrobiologia 160:53–61
Iwai N, Kagaya T (2005a) Difference in larval food habit of two Japanese Rana species. J Freshw Ecol 20:765–770
Iwai N, Kagaya T (2005b) Growth of Japanese toad (Bufo japonicus formosus) tadpoles fed different food items. Curr Herpetol 24:85–89
Iwai N, Kagaya T (2005c) Larval food habit of the forest green tree frog. Bull Herpetol Soc Japan 2005(2):100–102
Jones CG, Lawton JH, Shachak M (1997) Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78:1946–1957
Kupferberg S (1997) Facilitation of periphyton production by tadpole grazing: functional differences between species. Freshw Biol 37:427–439
Maeda N, Matsui M (1999) Frogs and toads of Japan. Bun-ichi Sogo Shuppan, Tokyo
McQueen DJ, Johannes MRS, Post JR, Stewart TJ, Lean DRS (1989) Bottom-up and top-down impacts on freshwater pelagic community structure. Ecol Monogr 59:289–309
Miyashita M, Yasuno M (1984) Growth and reproduction of Asellus hilgendorfii (Crustacea, Isopoda) under laboratory conditions. Jpn J Limnol 45:213–219
Mulholland PJ (1996) Role in nutrient cycling in streams. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology. Academic, New York, pp 609–639
Nolen JA, Pearson RG (1993) Factors affecting litter processing by Anisocentropus kirranus (Trichoptera: Calamoceratidae) from an Australian tropical rainforest stream. Freshw Biol 29:469–479
Oertli B (1993) Leaf litter processing and energy flow through macroinvertebrates in a woodland pond (Switzerland). Oecologia 96:466–477
Osborne PL, McLachlan AJ (1985) The effect of tadpoles on algal growth in temporary, rain-filled rock pools. Freshw Biol 15:77–87
Persson L, Bengtsson J, Menge BA, Power ME (1996) Productivity and consumer regulation—concepts, patterns, and mechanisms. In: Polis GA, Winemiller KO (eds) Food webs: integration of patterns and dynamics. Chapman & Hall, New York, pp 396–434
Peterson CG, Boulton AJ (1999) Stream permanence influences microalgal food availability to grazing tadpoles in arid-zone springs. Oecologia 118:340–352
Pieczyńska E (1986) Sources and fate of detritus in the shore zone of lakes. Aquat Bot 25:153–166
Pinowska A (2002) Effects of snail grazing and nutrient release on growth of the macrophytes Ceratophyllum demersum and Elodea canadensis and the filamentous green algae Cladophora sp. Hydrobiologia 479:83–94
Power ME (1990) Resource enhancement by indirect effects of grazers: armored catfish, algae, and sediment. Ecology 71:897–904
Pringle CM, Blake GA, Covich AP, Buzby KM, Finley A (1993) Effects of omnivorous shrimp in a montane tropical stream: sediment removal, disturbance of sessile invertebrates and enhancement of understory algal biomass. Oecologia 93:1–11
Rosemond AD, Pringle CM, Ramírez A, Paul MJ (2001) A test of top-down and bottom-up control in a detritus-based food web. Ecology 82:2279–2293
Seale DB (1980) Influence of amphibian larvae on primary production, nutrient flux, and competition in a pond ecosystem. Ecology 61:1531–1550
Steinwascher K, Travis J (1983) Influence of food quality and quantity on early larval growth of two anurans. Copeia 1983(1):238–242
Stelzer RS, Heffernan J, Likens GE (2003) The influence of dissolved nutrients and particulate organic matter quality on microbial respiration and biomass in a forest stream. Freshw Biol 48:1925–1937
Sterner RW (1986) Herbivores’ direct and indirect effects on algal populations. Science 231:605–607
Suberkropp K (1992) Interactions with invertebrates. In: Bärlocher F (ed) The ecology of aquatic hyphomycetes (Ecological studies 94). Springer, New York, pp 118–133
Suberkropp K (1998a) Microorganisms and organic matter decomposition. In: Naiman RJ, Bilby RE (eds) River ecology and management. Springer, New York, pp 120–143
Suberkropp K (1998b) Effect of dissolved nutrients on two aquatic hyphomycetes growing on leaf litter. Mycol Res 102:998–1002
Suberkropp K, Arsuffi TL (1984) Degradation, growth, and changes in palatability of leaves colonized by six aquatic hyphomycete species. Mycologia 76:398–407
Suberkropp K, Godshalk GL, Klug MJ (1976) Changes in the chemical composition of leaves during processing in a woodland stream. Ecology 57:720–727
Uthicke S (2001) Nutrient regeneration by abundant coral reef holothurians. J Exp Mar Biol Ecol 265:153–170
Vanni MJ (1996) Nutrient transport and recycling by consumers in lake food webs: implications for algal communities. In: Polis GA, Winemiller KO (eds) Food webs: integration of patterns and dynamics. Chapman & Hall, New York, pp 81–95
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370
Vanni MJ, Flecker AS, Hood JM, Headworth JL (2002) Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecol Lett 5:285–293
Webster JR, Benfield EF, Ehrman TP, Schaeffer MA, Tank JL, Hutchens JJ, D’Angelo DJ (1999) What happens to allochthonous material that falls into streams? A synthesis of new and published information from Coweeta. Freshw Biol 41:687–705
Wetzel RG (2001) Limnology: lake and river ecosystems. Academic, New York
Wilhelm FM, Hudson JJ, Schindler DW (1999) Contribution of Gammarus lacustris to phosphorus recycling in a fishless alpine lake. Can J Fish Aquat Sci 56:1679–1686
Winemiller KO, Polis GA (1996) Food webs: what can they tell us about the world? In: Polis GA, Winemiller KO (eds) Food webs: integration of patterns and dynamics. Chapman & Hall, New York, pp 1–22
Acknowledgments
We thank Mitsuhiko Toda for advice, Masahiko Tanahashi for help with measuring snout-vent lengths on the computer, Takashi Nakada for identifying algae, and Ross A. Alford for giving comments on our draft. We also thank Masayo Iwai, Takayuki Matsuo, and colleagues of the Laboratory of Forest Zoology at the University of Tokyo for help with the fieldwork and with care of the tadpoles.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl.
Rights and permissions
About this article
Cite this article
Iwai, N., Kagaya, T. Positive indirect effect of tadpoles on a detritivore through nutrient regeneration. Oecologia 152, 685–694 (2007). https://doi.org/10.1007/s00442-007-0682-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0682-6