Abstract
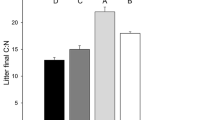
Decomposing leaf litter in streams provides habitat and nutrition for aquatic insects. Despite large differences in the nutritional qualities of litter among different plant species, their effects on aquatic insects are often difficult to detect. We evaluated how leaf litter of two dominant riparian species (Populus fremontii and P. angustifolia) influenced carbon and nitrogen assimilation by aquatic insect communities, quantifying assimilation rates using stable isotope tracers (13C, 15N). We tested the hypothesis that element fluxes from litter of different plant species better define aquatic insect community structure than insect relative abundances, which often fail. We found that (1) functional communities (defined by fluxes of carbon and nitrogen from leaf litter to insects) were different between leaf litter species, whereas more traditional insect communities (defined by relativized taxa abundances) were not different between leaf litter species, (2) insects assimilated N, but not C, at a higher rate from P. angustifolia litter compared to P. fremontii, even though P. angustifolia decomposes more slowly, and (3) the C:N ratio of material assimilated by aquatic insects was lower for P. angustifolia compared to P. fremontii, indicating higher nutritional quality, despite similar initial litter C:N ratios. These findings provide new evidence for the effects of terrestrial plant species on aquatic ecosystems via their direct influence on the transfer of elements up the food web. We demonstrate how isotopically labeled leaf litter can be used to assess the functioning of insect communities, uncovering patterns undetected by traditional approaches and improving our understanding of the association between food web structure and element cycling.




Similar content being viewed by others
References
Abdel-Raheem A, Shearer CA. 2002. Extracellular enzyme production by freshwater ascomycetes. Fungal Div 11:1–19.
Abdullah SK, Taj-Aldenn SJ. 1989. Extracellular enzymatic activity of aquatic and aero-aquatic conidial fungi. Hydrobiology 174:217–23.
Au DWT, Hodgkiss IJ, Vrijmoed LLP. 1991. Fungi and cellulolytic activity associated with decomposition of Bauhinia purpurea leaf litter in a polluted and unpolluted Hong Kong waterway. Can J Bot 70:1071–9.
Balseiro E, Albariño R. 2006. C–N mismatch in the leaf litter–shredder relationship of an Andean Patagonian stream detritivore. J N Am Benthol Soc 25:607–15.
Benfield EF. 2006. Decomposition of leaf material. In: Hauer FR, Lamberti GA, Eds. Methods in stream ecology. 2nd edn. Burlington (MA): Academic Press. p 125–55.
Bucher VVC, Pointing SB, Hyde KD, Reddy CA. 2004. Production of wood decay enzymes, loss of mass, and lignin solubilization in wood by diverse tropical freshwater fungi. Microbial Ecol 48:331–7.
Chamier AC. 1985. Cell-wall degrading enzymes of aquatic hyphomycetes: a review. Bot J Linn Soc 91:67–81.
Chapin FSIII, Matson PA, Mooney HA. 2002. Principles of terrestrial ecosystem ecology. New York (NY): Springer.
Clarke KR, Gorley RN. 2006. PRIMER v. 6: User manual/tutorial. Plymouth (MA): PRIMER-E.
Cross WF, Benstead JP, Rosemond AD, Wallace JB. 2003. Consumer-resource stoichiometry in detritus-based streams. Ecol Lett 6:721–32.
Cummins KW, Wiltzbach MA, Gates DM, Perry JB, Taliaferro WB. 1989. Shredders and riparian vegetation: leaf letter that falls into streams influences communities of stream invertebrates. Biosciences 39:24–31.
de Boer W, Folman LB, Summerbell RC, Boddy L. 2005. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29:795–811.
Driebe E, Whitham TG. 2000. Cottonwood hybridization affects tannin and nitrogen content of leaf litter and alters decomposition. Oecologia 123:99–107.
Dudgeon D, Gao BW. 2011. The influence of macroinvertebrate shredders, leaf type and composition on litter breakdown in a Hong Kong stream. Fundam Appl Limnol 178:147–57.
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA et al. 2000. Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–50.
Ensign SH, Doyle MW. 2006. Nutrient spiraling in streams and river networks. J Geophys Res Biogeosci. doi:10.1029/2005JG000114.
Eslyn WE, Moore WG. 1984. Bacterial wood decay accompanying deterioration in river pilings. Mater Org 19:263–82.
Evans-White MA, Stelzer RS, Lamberti GA. 2005. Taxonomic and regional patterns in benthic macroinvertebrate elemental composition in streams. Freshw Biol 50:1786–99.
Findlay S, Meyer JL, Smith PJ. 1986. Incorporation of microbial biomass by Peltoperla sp. (Plecoptera) and Tipula sp. (Diptera). J N Am Benthol Soc 5:306–10.
Fogel R, Cromack K Jr. 1977. The effect of habitat and substrate quality on Douglas-fir litter decomposition in western Oregon. Can J Bot 55:1632–40.
Friberg N, Jacobsen D. 1994. Feeding plasticity of two detritivore-shredders. Freshw Biol 32:133–42.
Friberg N, Jacobsen D. 1999. Variation in growth of the detritivore-shredder Sericostoma personatum (Trichoptera). Freshw Biol 42:625–35.
Frost PC, Tank SE, Turner MA, Elser JJ. 2003. Elemental composition of littoral invertebrates from oligotrophic and eutrophic Canadian lakes. J N Am Benthol Soc 22:51–62.
Fry B. 2008. Stable isotope ecology. New York: Springer.
Gauch HG Jr. 1982. Multivariate analysis and community structure. Cambridge: Cambridge University Press.
Golladay SW, Webster JR, Benfield EF. 1983. Factors affecting food utilization by a leaf shredding aquatic insect: leaf species and conditioning time. Holarct Ecol 6:157–62.
Gotelli NJ, Ellison AM. 2004. A primer of ecological statistics. Sunderland: Sinauer.
Grafius E, Anderson NH. 1979. Population dynamics, bioenergetics, and role of Lepidostoma quercina Ross (Trichoptera: Lepidostomatidae) in an Oregan woodland stream. Ecology 60:433–41.
Hendrix PF, Parmelee RW, Crossley DA, Coleman DC, Odum EP, Groffman PM. 1986. Detritus food webs in conventional and no-tillage agroecosystems. Biosciences 36:374–80.
Holeski LM, Hillstrom ML, Whitham TG, Lindroth RL. 2012. Relative importance of genetic, ontogenetic, induction and seasonal variation in producing a multivariate defense phenotype in a foundation tree species. Oecologia 170:695–707.
Holland EA, Coleman DC. 1987. Litter placement effects on microbial and organic matter dynamics in an agroecosystem. Ecology 68:425–33.
Holt DM, Jones EBG. 1983. Bacterial degradation of lignified wood cell walls in anaerobic aquatic habitats. Appl Environ Microbiol 46:722–7.
House HL. 1965. Effects of low levels of the nutrient content of a food and of nutrient imbalance on the feeding and nutrition of a phytophagous larva, Celerio euphorbiae. Can Entomol 97:62–8.
Iversen TM. 1979. Laboratory energetics of larvae of Sericostoma personatum (Trichoptera). Holarct Ecol 2:1–5.
Jacobsen D, Sand-Jensen K. 1994. Growth and energetics of a Trichopteran larva feeding on fresh submerged and terrestrial plants. Oecologia 97:412–18.
JMP Pro, v. 10. 2012. Cary (NC): SAS Institute Inc.
Kohlmeier S, Smits THM, Ford RM, Keel C, Harms H et al. 2005. Taking the fungal highway: mobilization of pollutant-degrading bacteria by fungi. Environ Sci Technol 39:4640–6.
Lawson DL, Klug MJ, Merritt RW. 1984. The influence of the physical, chemical and microbiological characteristics of decomposing leaves on the growth of the detritivore Tipula abdominalis (Diptera: Tipulidae). Can J Zool 62:2239–343.
Leake JR, Read DJ. 1997. Mycorrhizal fungi in terrestrial habitats. In: Wicklow DT, Söderström B, Eds. The mycota IV. Environmental and microbial relationships. Heidelberg: Springer. p 281.
LeRoy CJ, Marks JC. 2006. Litter quality, stream characteristics, and litter diversity influence decomposition rates and macroinvertebrates. Freshw Biol 51:605–17.
LeRoy CJ, Whitham TG, Keim P, Marks JC. 2006. Plant genes link forests and streams. Ecology 87:255–61.
LeRoy CJ, Whitham TG, Wooley SC, Marks JC. 2007. Within-species variation in foliar chemistry influences leaf-litter decomposition in a Utah river. J N Am Benthol Soc 26:426–38.
Li AOY, Ng LCY, Dudgeon D. 2009. Influence of leaf toughness and nitrogen content on litter breakdown and macroinvertebrates in a tropical stream. Aquat Sci 71:80–93.
Marks JC, Haden GA, Harrop BL, Reese EG, Keams JL et al. 2009. Genetic and environmental controls of microbial communities on leaf litter in streams. Freshw Biol 54:2616–27.
McCune B, Mefford MJ. 2011. PC-ORD, v. 6. Multivariate analysis of ecological data. Glenden Beach (OR): MjM Software.
McCune B, Grace JB, Urban DL. 2002. Analysis of ecological communities. Gleneden Beach (OR): MjM Software Design.
Melillo JM, Aber JD, Muratore JF. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–6.
Merritt RW, Cummins KW. 1996. An introduction to the aquatic insects of North America. Dubuque (IA): Kendall Hunt.
Mouchet MA, Villeger S, Mason NW, Mouillot D. 2010. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol 24:867–76.
Naeem S, Wright JP. 2003. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecol Lett 6:567–79.
Oertli B. 1993. Leaf litter processing and energy flow through macroinvertebrates in a woodland pond (Switzerland). Oecologia 96:466–77.
Otto C. 1974. Growth and energetics in a larval population of Potamophylax cingulatus (Steph.) (Trichoptera) in a South Swedish stream. J Animal Ecol 43:339–61.
Paine RT. 1980. Food webs: linkage, interaction strength and community infrastructure. J Anim Ecol 49:667–85.
Perry WB, Benfield EF, Perry SA, Webster JR. 1987. Energetics, growth, and production of a leaf-shredding stonefly in an Appalachian mountain stream. J N Am Benthol Soc 6:12–25.
Power ME. 1995. Floods, food chains, and ecosystem processes in rivers. In: Jones CG, Lawton JH, Eds. Linking species and ecosystems. New York: Chapman and Hall. p 52–60.
Schindler DW. 1971. A hypothesis to explain differences and similarities among lakes in the Experimental Lakes Area (ELA), northwestern Ontario. J Fish Res Board Can 28:295–301.
Schweitzer JA, Bailey JK, Rehill BJ, Martinsen GD, Hart SC et al. 2004. Genetically based trait in a dominant tree affects ecosystem processes. Ecol Lett 7:127–34.
Schweitzer JA, Bailey JK, Hart SC, Whitham TG. 2005a. Nonadditive effects of mixing cottonwood genotypes on litter decomposition and nutrient dynamics. Ecology 86:2834–40.
Schweitzer JA, Bailey JK, Hart SC, Wimp GM, Chapman SK et al. 2005b. The interaction of plant genotype and herbivory decelerate leaf litter decomposition and alter nutrient dynamics. Oikos 110:133–45.
Singh N. 1982. Cellulose decomposition by some tropical aquatic hyphomycetes. Trans Br Mycol Soc 79:560–1.
Singh AP, Butcher JA. 1991. Bacterial degradation of wood cell walls: a review of degradation patterns. J Inst Wood Sci 12:143–57.
Sokal RR, Rohlf FJ. 1995. Biometry: the principles and practice of statistics in biological research. 3rd edn. New York (NY): W. H. Freeman and Company.
Tibbets TM, Molles MC Jr. 2005. C:N:P stoichiometry of dominant riparian trees and arthropods along the Middle Rio Grande. Freshw Biol 50:1882–94.
Tuchman NC, Wetzel RG, Rier ST, Wahtera KA, Teeri JA. 2002. Elevated atmospheric CO2 lowers leaf litter nutritional quality for stream ecosystem food webs. Glob Change Biol 8:163–70.
van der Heijden MGA, Bardgett RD, van Straalen NM. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310.
Waldbauer GP. 1968. The consumption and utilization of food by insects. Adv Insect Physiol 5:229–89.
Wallace JB, Webster JR, Cuffney TF. 1982. Stream detritus dynamics: regulation by invertebrate consumers. Oecologia 53:197–200.
Watt AS. 1947. Pattern and process in the plant community. J Ecol 35:1–22.
Wymore AS, Compson ZG, Liu CM, Price LB, Whitham TG et al. 2013. Contrasting rRNA gene abundance patterns for aquatic fungi and bacteria in response to leaf-litter chemistry. Freshw Sci 32:663–72.
Wymore AS, Compson ZC, McDowell WH, Potter JD, Hungate BA, et al. 2014. Leaf litter dissolved organic carbon is distinct in composition and bioavailability to stream heterotrophs. Freshw Sci (under review).
Yuen TK, Hyde KD, Hodgkiss IJ. 1998. Physiological growth parameters and enzyme production in tropical freshwater fungi. Mater Org 32:2–16.
Yuen TK, Hyde KD, Hodgkiss IJ. 1999. Soft rot decay in tropical freshwater fungi. Mater Org 33:155–61.
Zare-Maivan H, Shearer CA. 1988. Extracellular enzyme production and cell wall degradation by freshwater lignicolous fungi. Mycologia 80:365–75.
Acknowledgments
We thank Greg Florian, Bradford Blake, and Philip Patterson for technical assistance with developing labeling chambers and greenhouse operations. The manuscript improved through insightful feedback from members of the Merriam-Powell Seminar for Research Design, the Cottonwood Ecology Group, and, in particular, Paul Dijkstra. The Merriam-Powell Center for Environmental Research provided laboratory space and statistical resources. NSF provided funding through the FIBR (DEB-0425908), IGERT (DGE-0549505), and Ecosystem Studies (DEB-1120343) research programs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
ZGC, BAH, JCM, GWK, and SCH contributed to designing the study. TGW established and maintained the common garden. ZGC and KJA designed and constructed labeling chambers, and ZGC, KJA, and JMM grew and harvested labeled leaves. BAH, GWK, and SCH assisted ZGC with developing labeling techniques. ZGC and BAH developed flux equations and ZGC, BAH, and JMM performed related analyses. ZGC wrote the first draft of the manuscript, and JCM, BAH, TGW, GWK, and SCH contributed substantially to revisions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Compson, Z.G., Hungate, B.A., Koch, G.W. et al. Closely Related Tree Species Differentially Influence the Transfer of Carbon and Nitrogen from Leaf Litter Up the Aquatic Food Web. Ecosystems 18, 186–201 (2015). https://doi.org/10.1007/s10021-014-9821-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-014-9821-1