Abstract
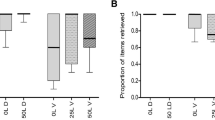
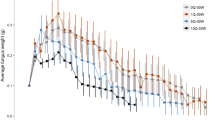
Myrmecochorous plant seeds have nutrient rich appendages, elaiosomes, which induce some ant species to carry the seeds back to their nest where the elaiosome is consumed and the seed is discarded unharmed. The benefits to plants of dispersal of their seeds in this way have been well documented, but the benefits to the ants from consuming the elaiosomes have rarely been measured and are less clear. Ant benefits from myrmecochory were investigated in a laboratory experiment using the ant Myrmica ruginodis and seeds of Ulex species. To separate the effects of elaiosome consumption on the development of newly produced larvae versus existing larvae, ten ‘Queenright’ colonies containing a queen were compared to ten ‘Queenless’ colonies. Six measures of colony fitness over a complete annual cycle were taken: sexual production, larval weight and number, pupal weight and number, and worker survival. Queenless colonies fed with elaiosomes produced 100.0±29.3 (mean ± SE) of larvae compared to non-elaiosome fed colonies which produced 49.6±19.0; an increase of 102%. Larval weight increased in both Queenright and Queenless colonies. In colonies fed with elaiosomes, larvae weighed 1.02±0.1 mg, but in non-elaiosome fed colonies larvae weighed 0.69±0.1 mg; an increase of 48%. The food supplement provided by Ulex elaiosomes was trivial in energetic terms, under the conditions of an ample diet, suggesting that these effects might be due to the presence of essential nutrients. Chemical analysis of Ulex elaiosomes showed the presence of four essential fatty acids and four essential sterols for ants.
Similar content being viewed by others
References
Andersen AA (1988) Patterns of ant community organisation in mesic south-eastern Australia. Aust J Ecol 11:87–97
Barbehenn RV, Reese JC, Hagens KS (1999) The food of insects. In: Huffaker CB, Gutierrez Ap (eds) Ecol Entomol. Wiley, New York
Beattie AJ (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press, Cambridge
Beattie AJ, Culver DC (1983) The nest chemistry of two seed-dispersing ant species. Oecologia 56:99–103
Begon M, Harper JL, Townsend CR (1996) Ecology. Blackwell, Oxford
Behmer ST, Elias DO, Grebenok RJ (1999) Phytosterol metabolism and absorption in the generalist grasshopper, Schistocerca Americana (Orthoptera: Acrididae). Arch Insect Biochem 42:13–25
Bluthgen N, Gottsberger G, Fiedler K (2004) Sugar and amino acid composition of ant- attended nectar and honeydew sources from an Austral rainforest. Aust Ecol 29:418–429
Bond WJ, Yeaton R, Stock WD (1991) Myrmecochory in Cape Fynbos. In: Huxley CR, Cutler DF (eds) Ant–plant interactions. Oxford Science publications, Oxford, pp 448–462
Bono JM, Heithaus ER (2002) Sex ratios and the distribution of elaiosomes in colonies of the ant, Aphaenogaster rudis. Insect Soc 49:320–325
Brew CR, O’Dowd DJ, Rae ID (1989) Seed dispersal by ants—behaviour-releasing compounds in elaiosomes. Oecologia 80:490–497
Brian MV (1977) Ants. Collins, London
Brian MV, Abbott A (1977) The control of food flow in a society of the ant Myrmica rubra L. Anim Behav 25:1047–1055
Brian MV, Rigby C (1978) The trophic eggs of Myrmica rubra L. Insect soc 25(1):89–110
Brown WD, Keller L, Sundström L (2002) Sex allocation in mound-building ants: The roles of resources and queen replenishment. Ecology 83:1945–1952
Bullock JM (2000) Geographical separation of two Ulex species at three spatial scale: does competition limit species range. Ecography 23:257–271
Christian CE (2001) Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 413:635–638
Christie WW (2003) Lipid analysis: Isolation, separation, identification and structural analysis of lipids, 3rd edn. Oily, Bridgwater
Dadd RH (1977) Handbook series in nutrition and food. CRC, Ohio
Elmes GW (1989) The effect of multiple queens in small groups of Myrmica rubra L. Insect Soc 5:137–144
Elmes GW (1991) The social biology of Myrmica ants. Insect Soc 7:17–34
Elmes GW, Wardlaw JC (1981) The quantity and quality of overwintered larvae in five species of Myrmica (Hymenoptera: Formicidae). J Zool 193:429–446
Elmes GW, Wardlaw JC (1983) A comparison of the effect of temperature on the development of large hibernated larvae of four species of Myrmica (Hym. Formicidae). Insect Soc 30:106–118
Elmes GW, Wardlaw JC, Schönrogge K, Thomas JA, Clarke RT (2004) Food stress causes differential survival of socially parasitic caterpillars of Maculinea rebeli integrated in colonies of host and non-host Myrmica ant species. Entomol Exp Appl 110:53–63
Espadaler X, Gómez C (1997) Soil surface searching and transport of Euphorbia characias seeds by ants. Acta Oecologica 18:39–46
Ferkovich SM, Shapiro J, Carpenter J (2000) Growth of a pupal ectoparasitoid, Diapetimorpha introita, on an artificial diet: stimulation of growth rate by a lipid extract from host pupae. Biocontrol 45:401–413
Fiedler K, Samm C (1995) Ants benefit from attending facultatively myrmecophilous Lycaenidae caterpillars: evidence form a survival study. Oecologia 104:316–322
Fischer RC, Richter A, Wanek W, Mayer V (2002) Plants feed ants: food bodies of myrmecophytic Piper and their significance for the interaction with Pheidole bicornis ants. Oecologia 133:186–192
Gómez C, Espadaler X (1998) Myrmecochorous dispersal distances: a world survey. J Biogeogr 25:573–580
Hagen KS, Dadd RH, Reese JC (1984) The food insects. In: Huffaker CB (eds) Ecological entomology. Wiley and Sons, New York
Handel SN, Beattie AJ (1990) Seed dispersal by ants. Sci Am 263:76
Heil M, Baumann B, Krüger R, Linsenmair KE (2004) Main nutrient compounds in food bodies of Mexican Acacia ant–plants. Chemoecology 14:45–52
Heinze J, Hölldobler B, Peeters C (1994) Conflict and cooperation in ant societies. Naturwissenschaften 81:489–497
Holbrook SJ, Schmitt RJ (2004) Population dynamics of a damselfish: effects of a competitor that also is an indirect mutualist. Ecology 85:979–985
Hölldobler B, Wilson EO (1990) The ants. Belknap, Harvard
Horvitz CC (1981) Analysis of how ant behaviours affect germination in a tropical myrmecochore Calathea microcephala (P.& E.) Koernicke (Mantaceae): microsite selection and aril removal by neotropical ants, Odontomachus, Pachycondyla, and Solenopsis (Formicidae). Oecologia 51:47–52
Hughes L, Westoby M (1992) Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 73:1300–1312
Kaluzny MA, Duncan LA, Merritt MV, Epps De (1985) Rapid separations of lipid classes in high yield and purity using bonded phase columns. J Lipid Res 26:135–140
Kusmenoglu S, Rockwood LL, Gretz MR (1989) Fatty acids and diacylglycerols from elaiosomes of some ant- dispersed seeds. Phytochemistry 28:2601–2602
Lanza J, Schmitt MA, Awad AB (1992) Comparative chemistry of elaiosomes of three species of Trillium. J Chem Ecol 18:209–221
Marshall DL, Beattie AJ, Bollenbacher WE (1979) Evidence for diglycerides as attractants in an ant-seed interaction. J Chem Ecol 5:335–344
Morales MA, Heithaus ER (1998) Food from seed-dispersal mutualism shifts sex ratios in colonies of the ant Aphaenogaster rudis. Ecology 79:734–739
Ohkawara K, Higashi S, Ohara M (1996) Effects of ants, ground beetles and the seed-fall patterns on myrmecochory of Erythronium japonicum Decne (Liliaceae). Oecologia 106:500–506
Raine NE, Gammans N, MacFadyen IJ, Scrivner GK, Stone GN (2004) Guards and thieves: antagonistic interactions between two ant species coexisting on the same ant-plant. Ecol Entomol 29:345–352
Rock GC (1985) The essential dietary fatty acid requirements of the tufted apple budmoth, Platynota idaensalis. J Insect Physiol 31:9–13
Rudgers JA, Gardener MC (2003) Extrafloral nectar as a resource mediating multispecies interaction. Ecology 85:1495–1502
Ryan BF, Joiner BL, Ryan T (2000) Minitab handbook, 4th edn. Brooks Cole, Florence
Schmitt RJ, Holbrook SJ (2004) Mutualism can mediate competition and promote coexistence. Ecol Lett 6:898–902
Sheridan SL, Iversen KA, Itagakis H (1996) The role of chemical senses in seed-carrying behaviour by ants: A behavioural, physiological and morphological study. J Insect Physiol 42:149–159
Smeeton L (1981) The source of males in Myrmica rubra L. (Hym. Formcidae). Insect Soc 28(3):263–278
Smeeton L (1982) The effects of the sizes of colony worker and food store on the production of reproductive eggs by workers of Myrmica Rubra L. (Hym. Formicidae). Insect Soc 29:475–484
Stokes KE, Bullock JM, Watkinson AR (2003) Ulex gallii Planch and Ulex minor Roth. J Ecol 91:1106–1124
Wardlaw JC, Elmes GW (1996) Exceptional colony size in Myrmica species (Hymenoptera: Formicidea). The Entomol 115:191–196
Wilson MF (1993) Dispersal mode, seed shadows, and colonization patterns. Vegetation 108:261–280
Zettler AJ, Spira TP, Allen CR (2001) Ant-seed mutualisms: can red imported fire ants sour the relationship. Biol Conserv 101:249–253
Acknowledgements
This experiment and methodology complies with the current laws of the UK. We would like to thank Graham Elmes, Judith Wardlaw and Michael Fenner for their advice and helpful criticism, Sophie Everett for her advice on chemistry and all the students who helped feed the colonies. This study was funded by the UK Natural Environment Research Council, research studentship to Nicola Gammans, NER/S/A/2002/11078.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bernhard Stadler
Rights and permissions
About this article
Cite this article
Gammans, N., Bullock, J.M. & Schönrogge, K. Ant benefits in a seed dispersal mutualism. Oecologia 146, 43–49 (2005). https://doi.org/10.1007/s00442-005-0154-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0154-9