Abstract
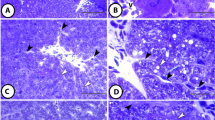
Peroxisomes of the hepatocytes of gray mullets, Mugil cephalus, were characterized cytochemically and immunocytochemically using antibodies against the peroxisomal proteins catalase and palmitoyl-coenzyme A (CoA) oxidase. In addition, morphometric parameters of peroxisomes were investigated depending on the hepatic zonation, the age of the animals and the sampling season. Mullet liver peroxisomes were reactive for diaminobenzidine, but presented a marked heterogeneity in staining intensity. Most of the peroxisomes were spherical or oval in shape, although irregular forms were also observed. Their size was heterogeneous, with profile diameters ranging from 0.2 to 3 µm. Peroxisomes tended to occur in clusters, usually near the mitochondria and lipid droplets. They also showed a very close topographical relationship to the smooth endoplasmic reticulum. Mullet liver peroxisomes did not contain cores or nucleoids as rodent liver peroxisomes, but internal substructures were observed in the matrix, consisting of small tubules about 60 nm in diameter and larger semicircles 120 nm in diameter. The volume density of peroxisomes was higher in periportal hepatocytes of mullets sampled in summer than in pericentral hepatocytes, indicating that mullet peroxisomes vary depending on physiological and environmental conditions. By immunoblotting, the mammalian antibodies cross-react with the corresponding proteins in whole homogenates of mullet liver. Paraffin sections immunostained with the antibodies against catalase and palmitoyl-CoA oxidase showed a positive reaction corresponding to peroxisomes localized in the hepatocyte cytoplasm. In agreement, the ultrastructural study revealed that catalase and palmitoyl-CoA oxidase are exclusively localized in the peroxisomal matrix in fish hepatocytes, showing a dense gold labeling. The presence of the peroxisomal β-oxidation enzyme palmitoyl-CoA oxidase in peroxisomes indicated that these organelles play a key role in the lipid metabolism of fish liver.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 25 January 1999 / Accepted: 27 April 1999
Rights and permissions
About this article
Cite this article
Orbea, A., Beier, K., Völkl, A. et al. Ultrastructural, immunocytochemical and morphometric characterization of liver peroxisomes in gray mullet, Mugil cephalus . Cell Tissue Res 297, 493–502 (1999). https://doi.org/10.1007/s004410051376
Issue Date:
DOI: https://doi.org/10.1007/s004410051376