Abstract
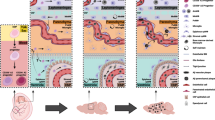
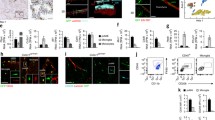
The dura mater contains abundant macrophages whose functions remain largely elusive. Recent studies have demonstrated the origin, as well as the gene expression pattern, of dural macrophages (dMΦs). However, their histological features have not been explored yet. In this study, we performed immunohistochemistry and electron microscopy to elucidate their precise morphology, localization, and postnatal development in mice. We found that the morphology, as well as the localization, of dMΦs changed during postnatal development. In neonatal mice, dMΦ exhibited an amoeboid morphology. During postnatal development, their cell bodies elongated longitudinally and became aligned along dural blood vessels. In adulthood, nearly half of the dMΦs aligned along blood vessel networks. However, most of these cells were not directly attached to vessels; pericytes and fibroblasts interposed between dMΦs and vessels. This morphological information may provide further indications for the functional significance of dMΦs.







Similar content being viewed by others
References
Ajami B, Bennett JL, Krieger C et al (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10:1538–1543
Andres KH, von During M, Muszynski K et al (1987) Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 175:289–301
Armulik A, Genove G, Mae M et al (2010) Pericytes regulate the blood-brain barrier. Nature 468:557–561
Cao Z, Lis R, Ginsberg M et al (2016) Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med 22:154–162
Cengiz P, Zafer D, Chandrashekhar JH et al (2019) Developmental differences in microglia morphology and gene expression during normal brain development and in response to hypoxia-ischemia. Neurochem Int 127:137–147
Chen S, Tisch N, Kegel M et al (2017) CNS macrophages control neurovascular development via CD95L. Cell Rep 19:1378–1393
Coles JA, Myburgh E, Brewer JM et al (2017) Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog Neurobiol 156:107–148
Derecki N, Derecki N, Kipnis J (2014) Mouse meninges isolation for FACS. Protoc Exch
Dimlich RV, Keller JT, Strauss TA et al (1991) Linear arrays of homogeneous mast cells in the dura mater of the rat. J Neurocytol 20:485–503
Filiano AJ, Xu Y, Tustison NJ et al (2016) Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535:425–429
Frederick N, Louveau A (2019) Meningeal lymphatics, immunity and neuroinflammation. Curr Opin Neurobiol 62:41–47
Ginhoux F, Greter M, Leboeuf M et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Sci 330:841–845
Goldmann T, Wieghofer P, Jordao MJ et al (2016) Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol 17:797–805
He H, Mack JJ, Guc E et al (2016) Perivascular macrophages limit permeability. Arterioscler Thromb Vasc Biol 36:2203–2212
Hoeffel G, Ginhoux F (2018) Fetal monocytes and the origins of tissue-resident macrophages. Cell Immunol 330:5–15
Jacobson S, Marcus EM, SpringerLink (Online service) (2011) Neuroanatomy for the neuroscientist. Springer US : Imprint: Springer,, Boston, MA, pp 1 online resource (XXIV, 404 p
Jordana M, Sarnstrand B, Sime PJ et al (1994) Immune-inflammatory functions of fibroblasts. Eur Respir J 7:2212–2222
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Kierdorf K, Erny D, Goldmann T et al (2013) Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16:273–280
Kierdorf K, Masuda T, Jordao MJC, Prinz M (2019) Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci 20:547–562
Konishi H, Okamoto T, Hara Y et al (2020) Astrocytic phagocytosis is a compensatory mechanism for microglial dysfunction. EMBO J e104464
Lapenna A, De Palma M, Lewis CE (2018) Perivascular macrophages in health and disease. Nat Rev Immunol 18:689–702
Leid J, Carrelha J, Boukarabila H et al (2016) Primitive embryonic macrophages are required for coronary development and maturation. Circ Res 118:1498–1511
Lenz KM, Nelson LH (2018) Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front Immunol 9:698
Louveau A, Smirnov I, Keyes TJ et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341
McIlvried LA, Cruz JA, Borghesi LA et al (2017) Sex-, stress-, and sympathetic post-ganglionic-dependent changes in identity and proportions of immune cells in the dura. Cephalalgia 37:36–48
McMenamin PG, Wealthall RJ, Deverall M et al (2003) Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res 313:259–269
Mildner A, Schmidt H, Nitsche M et al (2007) Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 10:1544–1553
Monteran L, Erez N (2019) The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol 10:1835
Protasoni M, Sangiorgi S, Cividini A et al (2011) The collagenic architecture of human dura mater. J Neurosurg 114:1723–1730
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Rua R, Lee JY, Silva AB et al (2019) Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nat Immunol 20:407–419
Schaeuble K, Britschgi MR, Scarpellino L et al (2017) Perivascular fibroblasts of the developing spleen act as LTalpha1beta2-dependent precursors of both T and B zone organizer cells. Cell Rep 21:2500–2514
Schain AJ, Melo-Carrillo A, Borsook D et al (2018) Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann Neurol 83:508–521
Schulz C, Gomez Perdiguero E, Chorro L et al (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Sci 336:86–90
Tay TL, Mai D, Dautzenberg J et al (2017) A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 20:793–803
Van Hove H, Martens L, Scheyltjens I et al (2019) A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22:1021–1035
Wake H, Moorhouse AJ, Jinno S et al (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29:3974–3980
Yamazaki T, Mukouyama YS (2018) Tissue specific origin, development, and pathological perspectives of pericytes. Front Cardiovasc Med 5:78
Zhang W, Dai M, Fridberger A et al (2012) Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A 109:10388–10393
Acknowledgments
We are grateful to Mr. K. Itakura for his contribution to the electron microscopy experiments, Ms. Y. Konishi for experimental arrangements; Ms. Y. Itai, Ms. K. Muraki, and Ms. N. Tawarayama for their technical assistance; and Ms. A. Asano for secretarial works. We also wish to acknowledge the Division for Medical Research Engineering, Nagoya University Graduate School of Medicine, for the usage of the electron microscope and Imaris software.
Funding
This work was partly supported by KAKENHI (“Grant-in-Aid for Scientific Research (B)” 19H03395 to H. Kiyama and “Grant-in-Aid for Scientific Research (C)” 19K06904 to H. Konishi) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and grants from The Ichiro Kanehara Foundation to H. Konishi.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiments complied with the ARRIVE guidelines and carried out in accordance with National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Also, our experimental procedures were conducted in accordance with standard guidelines for animal experiments from the Nagoya University Graduate School of Medicine, and all protocols were approved by the Institutional Animal Committees (approval numbers: 20217, 28303, 29281, 30178, and 31072).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, T., Konishi, H., Tamada, H. et al. Morphology, localization, and postnatal development of dural macrophages. Cell Tissue Res 384, 49–58 (2021). https://doi.org/10.1007/s00441-020-03346-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03346-y