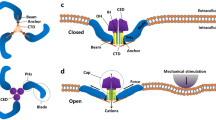
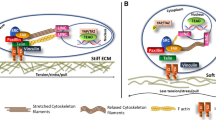
Abstract
Differentiation of stem cells can be modulated by a combination of internal and external signals, including mechanical cues from the surrounding microenvironment. Although numerous chemical and biological agents have been recognized in regulating stem cells’ fate, little is known about their potential to directly sense the mechanical signals to choose differentiation into a specific lineage. The success of any stem cell transplantation effort, however, hinges on thorough understanding of the fate of these cells under different signals, including mechanical cues. Various proteins are involved in the mechanical sensing process. Of these, Piezo proteins, as the ion channels activated by membrane tension and mechanical signals, play an important role in translating the information of mechanical forces such as rigidity and topography of the extracellular matrix to the intracellular signaling pathways related to stem cell homing and differentiation. They also play a key role in terms of shear stresses and tensile loads in expansion systems. This review highlights key evidence for the potential of mechanically gated ion channels expressed by human stem cells, and the mechanotransduction and past mechanomemory in the fate of transplanted stem cells. With this knowledge in mind, by controlling the tissue-specific patterns of mechanical forces in the scaffolds, we may further improve the regulation of homing, the differentiation, and the fate of transplanted stem cells.




Similar content being viewed by others
References
Anderson EO, Schneider ER, Matson JD, Gracheva EO, Bagriantsev SN (2018) TMEM150C/Tentonin3 is a regulator of mechano-gated ion channels. Cell Rep 23:701–708
Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae S-K, Kittappa R, McKay RDG (2006) Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442:823–826
Arteshi Y, Aghanejad A, Davaran S, Omidi Y (2018) Biocompatible and electroconductive polyaniline-based biomaterials for electrical stimulation. Eur Polym J 108:150–170
Arteshi Y, Aghanejad A, Davaran S, Omidi Y (2019) Semi self-doped electroconductive and biocompatible polyaniline/sulfonated β-cyclodextrin (PANI/SCD) inclusion complex with potential use in regenerative medicine. Int J Polym Mater Polym Biomater 69(7):437–448
Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S (2012) Role of TAZ as mediator of Wnt signaling. Cell 151:1443–1456
Bagriantsev SN, Gracheva EO, Gallagher PG (2014a) Piezo proteins: regulators of mechanosensation and other cellular processes. J Biol Chem R114:612697
Bagriantsev SN, Gracheva EO, Gallagher PG (2014b) Piezo proteins: regulators of mechanosensation and other cellular processes. J Biol Chem 289:31673–31681
Barzegari A, Gueguen V, Omidi Y, Ostadrahimi A, Nouri M, Pavon-Djavid G (2020) The role of hippo signaling pathway and mechanotransduction in tuning embryoid body formation and differentiation. J Cell Physiol 235(6):5072–5083. https://doi.org/10.1002/jcp.29455
Beech DJ, Caolo V, Debant M, Endesh N, Futers S, Lichtenstein L, Parsonage G, Jones EA (2019) Piezo1 channel activates ADAM10 sheddase to regulate Notch1 and gene expression. BioRxiv 732370. https://doi.org/10.1101/732370
Buyan A, Cox CD, Rae J, Barnoud J, Li J, Cvetovska J, Bastiani M, Chan HS, Hodson MP, Martinac B (2019) Piezo1 induces local curvature in a mammalian membrane and forms specific protein-lipid interactions. bioRxiv 787531. https://doi.org/10.1101/787531
Chachisvilis M, Zhang Y-L, Frangos JA (2006) G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci 103:15463–15468
Clark EA, King WG, Brugge JS, Symons M, Hynes RO (1998) Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol 142:573–586
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60
Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483:176
Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng C-A, Sachs F, Gottlieb PA, Martinac B (2016) Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun 7:10366
Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO (2007) The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6:997
D'angelo F, Armentano I, Mattioli S, Crispoltoni L, Tiribuzi R, Cerulli G, Palmerini C, Kenny J, Martino S, Orlacchio A (2010) Micropatterned hydrogenated amorphous carbon guides mesenchymal stem cells towards neuronal differentiation. Eur Cell Mater 20:231–244
Discher DE, Mooney DJ, Zandstra PW (2009) Growth factors, matrices, and forces combine and control stem cells. Science 324:1673–1677
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179
Eaker S, Armant M, Brandwein H, Burger S, Campbell A, Carpenito C, Clarke D, Fong T, Karnieli O, Niss K, Van't Hof W, Wagey R (2013) Concise review: guidance in developing commercializable autologous/patient-specific cell therapy manufacturing. Stem Cells Transl Med 2:871–883
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689
Eyckmans J, Boudou T, Yu X, Chen CS (2011) A hitchhiker’s guide to mechanobiology. Dev Cell 21:35–47
Fathi M, Barar J, Aghanejad A, Omidi Y (2015) Hydrogels for ocular drug delivery and tissue engineering. BioImpacts 5:159
Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A (2004) Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol 6:977
Gao Q, Walmsley AD, Cooper PR, Scheven BA (2016) Ultrasound stimulation of different dental stem cell populations: role of mitogen-activated protein kinase signaling. J Endod 42:425–431
Gao Q, Cooper PR, Walmsley AD, Scheven BA (2017) Role of Piezo channels in ultrasound-stimulated dental stem cells. J Endod 43:1130–1136
Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM (2010) Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329:1078–1081
Gilchrist CL, Leddy HA, Kaye L, Case ND, Rothenberg KE, Little D, Liedtke W, Hoffman BD, Guilak F (2019) TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proc Natl Acad Sci 116:1992–1997
Gottlieb PA, Bae C, Sachs F (2012) Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels 6:282–289
Guerrero J, Oliveira H, Aid R, Bareille R, Siadous R, Letourneur D, Mao Y, Kohn J, Amédée J (2018) Influence of the three-dimensional culture of human bone marrow mesenchymal stromal cells within a macroporous polysaccharides scaffold on Pannexin 1 and Pannexin 3. J Tissue Eng Regen Med 12:e1936–e1949
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5:17–26
Guo YR, MacKinnon R (2017) Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife 6:e33660
Guo L, Cai T, Chen K, Wang R, Wang J, Cui C, Yuan J, Zhang K, Liu Z, Deng Y (2018) Kindlin-2 regulates mesenchymal stem cell differentiation through control of YAP1/TAZ. J Cell Biol 217:1431–1451
Hamill OP, Martinac B (2001) Molecular basis of mechanotransduction in living cells. Physiol Rev 81:685–740
He L, Si G, Huang J, Samuel AD, Perrimon N (2018) Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 555:103
Heathman TR, Glyn VA, Picken A, Rafiq QA, Coopman K, Nienow AW, Kara B, Hewitt CJ (2015) Expansion, harvest and cryopreservation of human mesenchymal stem cells in a serum-free microcarrier process. Biotechnol Bioeng 112:1696–1707
Heo SJ, Szczesny SE, Kim DH, Saleh KS, Mauck RL (2018) Expansion of mesenchymal stem cells on electrospun scaffolds maintains stemness, mechano-responsivity, and differentiation potential. J Orthop Res 36:808–815
Holle AW, Tang X, Vijayraghavan D, Vincent LG, Fuhrmann A, Choi YS, Álamo JC, Engler AJ (2013) In situ mechanotransduction via vinculin regulates stem cell differentiation. Stem Cells 31:2467–2477
Hong J-H, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA (2005) TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309:1074–1078
Ingber DE (2006) Cellular mechanotransduction: putting all the pieces together again. FASEB J 20:811–827
Isomursu A, Lerche M, Taskinen M, Ivaska J, Peuhu E (2019) Integrin signaling and mechanotransduction in regulation of somatic stem cells. Exp Cell Res 378(2):217–225
Janaszak M, Wolfe R, Ahsan T (2016) Biomechanics in stem cell manufacturing. In: Stem cell manufacturing. Elsevier, pp 27–42. https://doi.org/10.1016/B978-0-444-63265-4.00002-9
Johnson CP, Tang H-Y, Carag C, Speicher DW, Discher DE (2007) Forced unfolding of proteins within cells. Science 317:663–666
Katsumi A, Orr AW, Tzima E, Schwartz MA (2004) Integrins in mechanotransduction. J Biol Chem 279:12001–12004
Kilian KA, Bugarija B, Lahn BT, Mrksich M (2010) Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci 107:4872–4877
Kim TJ, Seong J, Ouyang M, Sun J, Lu S, Hong JP, Wang N, Wang Y (2009) Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J Cell Physiol 218:285–293
Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, da Costa FL, Guck J (2016) Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci 19:1592
Kropp C, Massai D, Zweigerdt R (2017) Progress and challenges in large-scale expansion of human pluripotent stem cells. Process Biochem 59:244–254
Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT (2004) Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 113:370–378
Lee J, Abdeen AA, Zhang D, Kilian KA (2013) Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 34:8140–8148
Lee J-H, Kim D-H, Lee H-H, Kim H-W (2019) Role of nuclear mechanosensitivity in determining cellular responses to forces and biomaterials. Biomaterials 197:60–71
Lewis AH, Grandl J (2015) Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife 4:e12088
Liu T, Shindel AW, Lin G, Lue TF (2019) Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int J Impot Res 31:170–176
Ma Y, Lin M, Huang G, Li Y, Wang S, Bai G, Lu TJ, Xu F (2018) 3D spatiotemporal mechanical microenvironment: a hydrogel-based platform for guiding stem cell fate. Adv Mater 30:1705911
MacQueen L, Sun Y, Simmons CA (2013) Mesenchymal stem cell mechanobiology and emerging experimental platforms. J R Soc Interface 10:20130179
Marchant JS (2019) Ca2+ signaling and regeneration. Cold Spring Harb Perspect Biol 11(11). https://doi.org/10.1101/cshperspect.a035485
Marrelli M, Codispoti B, Shelton RM, Scheven BA, Cooper PR, Tatullo M, Paduano F (2018) Dental pulp stem cell mechanoresponsiveness: effects of mechanical stimuli on dental pulp stem cell behavior. Front Physiol 9:1685. https://doi.org/10.3389/fphys.2018.01685
McHugh BJ, Buttery R, Lad Y, Banks S, Haslett C, Sethi T (2010) Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J Cell Sci 123:51–61
Meyers VE, Zayzafoon M, Gonda SR, Gathings WE, McDonald JM (2004) Modeled microgravity disrupts collagen I/integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. J Cell Biochem 93:697–707
Meyers VE, Zayzafoon M, Douglas JT, McDonald JM (2005) RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res 20:1858–1866
Mokhtarzadeh A, Alibakhshi A, Hejazi M, Omidi Y, Dolatabadi JEN (2016) Bacterial-derived biopolymers: advanced natural nanomaterials for drug delivery and tissue engineering. TrAC Trends Anal Chem 82:367–384
Murphy WL, McDevitt TC, Engler AJ (2014) Materials as stem cell regulators. Nat Mater 13:547
Nourse JL, Pathak MM (2017) How cells channel their stress: interplay between Piezo1 and the cytoskeleton. Semin Cell Dev Biol 71. Elsevier:3–12
Pardo-Pastor C, Rubio-Moscardo F, Vogel-González M, Serra SA, Afthinos A, Mrkonjic S, Destaing O, Abenza JF, Fernández-Fernández JM, Trepat X (2018) Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc Natl Acad Sci 115:1925–1930
Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Dai Trang TL, Bernardis E, Flanagan LA, Tombola F (2014) Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci 111:16148–16153
Perestrelo T, Correia M, Ramalho-Santos J, Wirtz D (2018) Metabolic and mechanical cues regulating pluripotent stem cell fate. Trends Cell Biol 28(12):1014–1029
Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M, Tauc M, Duranton C, Paulais M, Teulon J (2013) Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep 14:1143–1148
Poudineh M, Wang Z, Labib M, Ahmadi M, Zhang L, Das J, Ahmed S, Angers S, Kelley SO (2018) Three-dimensional nanostructured architectures enable efficient neural differentiation of mesenchymal stem cells via Mechanotransduction. Nano Lett 18:7188–7193
Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M, Hu J (2015) Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat Commun 6:8512
Raouf R, Lolignier S, Sexton JE, Millet Q, Santana-Varela S, Biller A, Fuller AM, Pereira V, Choudhary JS, Collins MO (2018) Inhibition of somatosensory mechanotransduction by annexin A6. Sci Signal 11:eaao2060
Re’em T, Cohen S (2011) Microenvironment design for stem cell fate determination. In: Tissue engineering III: cell-surface interactions for tissue culture, vol 126 Springer-Verlag, Berlin, Heidelberg, pp 227–262
Resnick A, Hopfer U (2007) Force-response considerations in ciliary mechanosensation. Biophys J 93:1380–1390
Riehl BD, Donahue HJ, Lim JY (2017) Fluid flow control of stem cells with investigation of mechanotransduction pathways. In: Biology and engineering of stem cell niches. Elsevier, pp 257–272. https://doi.org/10.1016/B978-0-12-802734-9.00017-2
Santos DM, Xavier JM, Morgado AL, Sola S, Rodrigues CM (2012) Distinct regulatory functions of calpain 1 and 2 during neural stem cell self-renewal and differentiation. PLoS One 7:e33468
Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB (2018) Structure of the mechanically activated ion channel Piezo1. Nature 554:481
Shi Y, Li H, Zhang X, Fu Y, Huang Y, Lui PPY, Tang T, Dai K (2011) Continuous cyclic mechanical tension inhibited Runx2 expression in mesenchymal stem cells through RhoA-ERK1/2 pathway. J Cell Physiol 226:2159–2169
Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T, Ueda-Yamaguchi K, Kitamura T, Yoshizaki K, Fukumoto S (2017) Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep 7:17696
Sukharev S, Corey DP (2004) Mechanosensitive channels: multiplicity of families and gating paradigms. Sci STKE 2004:re4–re4
Sun Z, Guo SS, Fässler R (2016) Integrin-mediated mechanotransduction. J Cell Biol 215:445–456
Syeda R (2017) Piezo1 channels are inherently mechanosensitive. Biophys J 112:8a
Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T (2001) Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron 29:45–55
Totaro A, Panciera T, Piccolo S (2018) YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 20:888
Uhlén P, Fritz N (2010) Biochemistry of calcium oscillations. Biochem Biophys Res Commun 396:28–32
VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å (2012) Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139:488–497
Vining KH, Mooney DJ (2017) Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 18:728
Viti F, Landini M, Mezzelani A, Petecchia L, Milanesi L, Scaglione S (2016) Osteogenic differentiation of MSC through calcium signaling activation: transcriptomics and functional analysis. PLoS One 11:e0148173
Watt FM, Huck WT (2013) Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol 14:467
Wu J, Lewis AH, Grandl J (2017) Touch, tension, and transduction–the function and regulation of Piezo ion channels. Trends Biochem Sci 42:57–71
Yajima Y, Kawashima S (2002) Calpain function in the differentiation of mesenchymal stem cells. Biol Chem 383:757–764
Yang C, Tibbitt MW, Basta L, Anseth KS (2014) Mechanical memory and dosing influence stem cell fate. Nat Mater 13:645
Zhang T, Chi S, Jiang F, Zhao Q, Xiao B (2017) A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat Commun 8:1797
Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong M-Q (2018) Structure and mechanogating mechanism of the Piezo1 channel. Nature 554:487
Funding
This study was funded by the Stem Cell and Regenerative Medicine Institute, Tabriz University of Medical Sciences (Ph.D. Thesis no: 95/4-5/6).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barzegari, A., Omidi, Y., Ostadrahimi, A. et al. The role of Piezo proteins and cellular mechanosensing in tuning the fate of transplanted stem cells. Cell Tissue Res 381, 1–12 (2020). https://doi.org/10.1007/s00441-020-03191-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03191-z