Abstract
Lipid droplet (LD) binding proteins in mammary glands and in adipocytes were previously compared and striking similar sets of these specific proteins demonstrated. Xanthine oxidoreductase (XOR) together with perilipins and the lactating mammary gland protein butyrophilin play an important role in the secretion process of LDs into milk ducts. In contrast, in adipose tissue and in adipocytes, mainly perilipins have been described. Moreover, XOR was reported in mouse adipose tissue and adipocyte culture cells as “novel regulator of adipogenesis”. This obvious coincidence of protein sets prompted us to revisit the formation of LDs in human-cultured adipocytes in more detail with special emphasis on the possibility of a LD association of XOR. We demonstrate by electron and immunoelectron microscopy new structural details on LD formation in adipocytes. Surprisingly, by immunological and proteomic analysis, we identify in contrast to previous data showing the enzyme XOR, predominantly the expression of aldehyde oxidase (AOX). AOX could be detected tightly linked to LDs when adipocytes were treated with starvation medium. In addition, the majority of cells show an enormous interconnected, tubulated mitochondria network. Here, we discuss that (1) XOR is involved—together with perilipins—in the secretion of LDs in alveolar epithelial cells of the lactating mammary gland and is important in the transcytosis pathway of capillary endothelial cells. (2) In cells, where LDs are not secreted, XOR cannot be detected at the protein level, whereas in contrast in these cases, AOX is often present. We detect AOX in adipocytes together with perilipins and find evidence that these proteins might direct LDs to mitochondria. Finally, we here report for the first time the exclusive and complementary localization of XOR and AOX in diverse cell types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the origin, the formation and the accumulation of lipid droplets (LDs) in a variety of different cells have been investigated intensely in order to contribute insights to a general problem: obesity as an increasing worldwide major health concern in modern life (Sztalryd and Kimmel 2014). Of particular interest were proteins of the perilipin family bound to LDs (Wang and Sztalryd 2011). The LDs in milk are surrounded by a proteinaceous coat, i.e., the milk lipid globule membrane (MLGM), comprise members of the perilipin family (PLIN2-3), the lactation-specific transmembrane protein butyrophilin (BTN1A) and xanthine oxidoreductase (XOR) as major LD-binding proteins (see Franke et al. 1981; Jarasch et al. 1981; Heid and Keenan 2005). During lactation, the enzyme XOR is involved in the secretion of LDs from the mammary epithelial cells into the mammary gland ducts. This enzyme was also found and localized in capillary endothelial cells (Jarasch et al. 1981; Jarasch et al. 1986; Bruder et al. 1984). In general, XOR regulates cellular redox and detoxification processes, representing an evolutionary old oxidative defense mechanism (Vorbach et al. 2003; Vorbach et al. 2006). XOR is also reported to be responsible for the final two steps in purine metabolism by converting hypoxanthine to xanthine and xanthine to uric acid with concomitant generation of reactive oxygen species (ROS). Generated ROS products are considered responsible for antimicrobial activity and are involved in various pathological situations (McCord et al. 1985; Berry and Hare 2004; Martin et al. 2004; Battelli et al. 2019).
In LDs isolated from adipocytes, specific protein members of the perilipin family are well established as major LD surface-associated proteins in association with the intermediate filament protein vimentin (Franke et al. 1987; Greenberg et al. 1991; Blanchette-Mackie et al. 1995; Wolins et al. 2005; Heid et al. 2014). Using gene profiling methods and a novel algorithm, XOR was reported (Cheung et al. 2007) as “regulator of adipogenesis and PPARy activity”. These striking similarity of sets of LD-associated proteins found in lactating mammary gland cells and in adipocytes prompted us to revisit early adipocyte differentiation in more detail, with special focus on XOR expression.
Here, we show by electron and immunoelectron microscopy new structural details on early droplet formation in cultured human adipocytes. Further, we identify and demonstrate the expression of aldehyde oxidase (AOX) by proteomic and biochemical analysis. We present evidence that AOX is primarily not participating in LD formation and LD packaging during adipogenesis but is involved in lipolysis processes by nutrition deprivation. Implications and possible functions of XOR for packaging and secretion of LDs in the lactating mammary gland versus AOX directing LDs to mitochondria of adipocytes, assisting ordered release of fatty acids for β-oxidation, are discussed.
Material and methods
Antibodies and reagents
The generation of monoclonal (mab) and polyclonal (pab) antibodies (abs) against members of the perilipin family of LD-binding proteins was recently described (Heid et al. 2013). New abs against butyrophilin (BTN1A), xanthine oxidoreductase (XOR) and aldehyde oxidase (AOX) were raised accordingly. These abs are summarized in Table S1 of Supplementary material. Additional abs used for testing and for controls in this study are also listed or given in previous publications (Heid et al. 2013; Heid et al. 2014).
Cell cultures
Human preadipose cells (Poietics from subcutaneous preadipocytes), growth media and adipocyte differentiation medium (ADM; also named adipocyte induction medium, AIM)—containing insulin, dexamethasone, indomethacin and isobutyl-methylxanthine—were obtained from Lonza (Basel, Switzerland; cat. # PT-5020, PT-8002 and PT-8202). Cell lysates were obtained as previously described (Heid et al. 2013).
Biochemical methods
Isolation of MLGM material was according to Franke et al. (Franke et al. 1981). For nitrogen cavitation, density gradient separation, SDS-PAGE, immunoblotting, immunofluorescence microscopy, immunoprecipitation and mass spectrometry analysis, see Heid et al. (2013).
Electron and immunoelectron microscopy
For electron microscopy (EM), cells were grown on cover slips and fixed as previously described using glutaraldehyde and OsO4—either simultaneously (Franke et al. 1969) or sequentially (Heid et al. 2013). For further details of immunoelectron microscopical techniques, see Franke et al. (1987).
Results
Expression of lipid droplet–associated proteins in human mammary gland and in adipocytes
We compared the proteins described in the literature, which encase LDs, secreted from epithelial cells in lactating mammary gland, with LDs obtained from mesenchymal-derived adipocytes and identified a high similarity of the major proteins (Fig. 1a). The major LD-associated proteins from isolated MLGMs comprise the lactation-specific, transmembrane protein butyrophilin (BTN), the more general LD-binding proteins perilipin 2 and 3 (PLIN2, PLIN3) and xanthine oxidoreductase (XOR). In preadipocytes, in converted and differentiated mature adipocytes, similarly to LDs derived from milk, specific perilipins (PLIN1–4) are associated to LD surfaces. In addition, we wanted to check whether the reported occurrence of XOR in adipocytes (Cheung et al. 2007) following the conversion of preadipocytes to adipocytes (Fig. 1b) is also LD associated. First, the XOR enzyme activity data during adipogenesis—reported by Cheung et al. (2007)—were compared with the expression data of perilipins described in our previous observations on adipocytes (Heid et al. 2014) (Fig. 1c).
Comparison of major lipid droplet (LD) binding proteins detected in milk vs. adipocytes, scheme for adipocyte differentiation and expression profile of LD binding proteins during differentiation complemented by reported XOR activity. a LD binding proteins found in epithelial derived milk lipid droplet membranes (MLGM; blue box) and in mesenchymal derived adipocytes (yellow box) are compared. The proteins listed, BTN, PLIN 1–4 and XOR, have been described in literature (for MLGM, see, e.g., Franke et al. 1981; Jarasch et al. 1981; Heid and Keenan 2005; for human adipocytes, see, e.g., Heid et al. 2014). Xanthine oxidase (XOR) activity was reported in adipocytes (Cheung et al. 2007). b Scheme for adipocyte differentiation. Treatments with adipose induction medium (AIM; days (d)) and additionally with oleic acid (OA; hours (h)) are indicated. Upon stimulation, LDs are endogenously generated at the endoplasmic reticulum and stained positively for perilipin 1 (PLIN1; red droplets). Other LDs originating mainly from uptake of lipids via an endocytotic process and stain positively for PLIN2–4 (green droplets). Merged droplets, by fusion of different PLIN staining of LDs, appear in yellow. c Relative expression of LD associated PLIN proteins compared with reported XOR activity during adipocyte differentiation. The profiles in this graph for the PLIN proteins are drawn schematically according to our own data of immunoblotting and of immunofluorescence microscopy experiments (green and red lines). The profile of XOR until “day 3” follows enzyme activity measurements given by Cheung et al. (2007) (blue line). No data on XOR activity are available after OA treatment; therefore, the extrapolation for XOR drawn after day 3 is unknown (dashed blue line)
Selecting suitable antibodies for cultured adipocytes
In order to test the adipocyte cell system for proper performance of the various antibodies (abs) for correct localization, we used low passages of human preadipocytes (schematically shown in Fig. 1b). Several of the commercial available abs have been proven unspecific and unreliable, e.g., one ab reacted positive in immunoblotting with a band at 27 kD, within the reference lane and turned out to be triosephosphate isomerase (Escherichia coli). This protein is completely unrelated to XOR or AOX (see Fig. S5). In recent years, false findings by abs are spread all over the field and the specificity of abs became a matter of intensive debate (Baker 2015). It is evident that proper validation of abs, including eventual cross-reactivity and other drawbacks, should be supplied. For example, one report on xanthine oxidase inhibition by febuxostat is stating for used ab only “XO (1:100, Abcam)” and no product number and no further information are given (Yisireyili et al. 2017). Another example with a company statement not very useful on an antigen source (“Peptide internal sequence: considered to be commercially sensitive”) is given in Table S1. Therefore, we characterized available XOR abs in parallel to immunofluorescence microscopy (IFM) by immunoblotting and obtained some conflicting and/or negative results (Fig. S5). To overcome this problem, we obtained additional commercial abs and further also generated a series of novel abs (Table S1). We tested and selected 7 different abs specific for PLIN, with 12 different abs specific for XOR and 7 different abs specific for aldehyde oxidase (AOX). Using these abs, we demonstrate exemplarily a wide heterogeneity of LD labeling (Fig. S1).
Electron microscopic studies on the endogenous formation of LDs
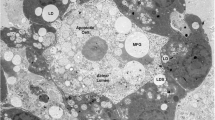
We extended our previous EM and immunelectron microscopy (IEM) studies on adipocytes and adipogenesis (Heid et al. 2014) and found endogenously emergence of very small LDs (sLDs) of forming droplets completely surrounded by vimentin intermediate filaments (“wide space type”) (Fig. S2a). The formation and assembly of distinct structures of endoplasmatic reticulum (ER) could be observed (Fig. S2b), structures that approach and encase smaller LDs. In the following steps of growing LDs, the emerging ER tubules, cisternae sheaths and layers were seen interconnected directly with vimentin intermediate filaments at the LDs (Fig. S3a,b). The filaments were arranged in a “high density spacing” modus and mitochondria are kept away from LDs by the intermediate filaments and the ER layers. Multiple ER layers were surrounding and wrapping LDs. The LDs became bigger and bigger in size; they “grow” during these special ER encasements (Fig. 2). Specific situations of ER layers and rare contacts of LDs with mitochondria could also occasionally be seen (Fig. S4a,b).
Electron micrographs showing LDs in human preadipocytes upon stimulation for adipocyte differentiation, revealing multiple staggered endoplasmatic reticulum (ER) layers that encase forming LDs completely. a, b Advanced droplet formation with growing LDs embedded in a dense net of intermediate filaments. Some of these intermediate filaments, seen in transverse sections, are marked by arrows. Multiple layers of ER cisternae encircle and wrap droplets completely. Examples with four to six ER layers are presented, respectively. LD lipid droplets, M mitochondria. Bars 500 nm
EM studies of LDs of adipocyte conversion and OA uptake
Upon addition of oleic acid (OA) to the culture medium, the sheaths of multiple ER cisternae and layers seen in earlier LD formation disappear rapidly. Many, very small microvesicles appear predominantly at the plasma membrane (Fig. 3a). Clusters of vesicle-like structures and invaginations with “rosette” structures, i.e., special spatial arrangements of microvesicles, represent further the high frequency of endocytotic uptake of lipids with the OA treatment (Fig. 3b). Ordered bundles of “high density spacing” vimentin filament surrounding LDs are replaced by loosely LD-attached filaments and “wide space type” filament arrangement; ER layers are fragmented and mitochondria are no more excluded by them and can approach LDs (Fig. 3c).
Electron micrographs showing LDs in human preadipocytes upon adipocyte conversion: additional treatment with oleic acid (OA) leads to many microvesicles and “rosette-like” structures. (a) With OA treatment, many small endocytotic vesicles and invaginations emerge in the cytoplasm near the plasma membrane, greatly increased in number (arrowheads). (a′) Three vesicles, seen in (a) in an endocytosis process, are shown in the enlarged insert (arrows). (b) Example of a cluster of vesicle-like structures (here named: vls; arrows) seen besides a rosette-like structure (asterisk) and LDs. “Rosettes” are special spatial compartments, flask-like invaginations of the plasma membrane with regularly arranged mircovesicles. They are involved in exogenous uptake of lipids (see Williamson 1964; Novikoff et al. 1980). (c) The multiple layers of ER cisternae surrounding LDs (also seen in Figs. 2 and S3) disappear upon OA treatment and ordered bundles of intermediate filaments surround LDs as surface-anchored “wide-space type” intermediate filaments (marked by light arrows). Other intermediate filaments connected to LDs and reaching out into the cytoplasm are also marked (black arrows). Some LDs are seen connected via intermediate filaments to mitochondria (M). Bars: (a) 1 μm and (a′, b, c) 500 nm
IEM localization studies of adipocyte conversion and uptake of OA
We next performed IEM localization studies using several of the novel abs (see Table S1) and demonstrated that the rims of ER-derived endogenous LDs in differentiating adipocytes showed a positive reaction for PLIN1. These LDs are loosely associated with bundles of intermediate filaments and at the beginning of conversion, are occasionally contacted by mitochondria (Fig. 4a). Upon addition of OA to the adipocyte culture medium, many microvesicle-like structures and a few contacts of LDs and mitochondria are observed (Fig. 4b). IEM for PLIN2 on differentiating adipocytes reveal several midsize LDs (LD; 200–500 nm in diameter) and clusters of sLD (50–125-nm sizes in diameter). In addition, most LD and sLD are connected by bundles of intermediate filaments (Fig. 5a). Unlabeled, considerably bigger LDs are seen more often (not shown here). An example of differently distinguishable LDs in adipocytes treated with OA is given for PLIN2 labeling in Fig. 5(b). Upon addition of OA, many microvesicle-like structures can be noticed; however, these invaginations of the plasma membrane are not labeled with PLINs.
Immunoelectron microscopic (IEM) localization of PLIN1 in human preadipocytes upon adipocyte conversion, followed by additional treatment with OA. a, b Positive reaction with monoclonal antibody mab Peri112.17 by nanogold labeling and silver enhancement found at the surface of endogenously, ER-derived LDs. These LDs are closely connected by a network of intermediate filaments (bold arrows). a Two LDs shown are in a situation of apparent immediate fusion. Between the adhesion sites of the droplets (large bracket), no structures are detectable, even a simple dot pattern of cross-sectioned intermediate filaments is missing. b With OA uptake, prominent plasmalemmal invaginations and smaller vesicle-like structures appear; many are seen in clusters (vls; regular arrows). These are not labeled for PLIN1 and feature no real vesicles inside the cytoplasm but rather originate from cross-sectioned invaginations built during uptake of OA. Figure 4(a) shows a very rare situation at the start of adipocyte conversion, an approach of a mitochondrion towards one LD (marked by a small bracket). These special mitochondria-LD contacts are seen in somewhat higher numbers with OA treatment in Fig. 4(b), i.e., when ER layers are suddenly disrupted. M mitochondria. Bars 500 nm
IEM localization of PLIN2 in human preadipocytes upon brief stimulation for adipocyte differentiation, followed by additional treatment with OA. a Using mab AP125, LD labeling is seen mostly at the surface of midsize droplets of several 100 nm in diameter. Collateral LD-anchored intermediate filaments are marked (bold arrows in Fig. 5a). Clusters of very small, positively labeled droplets can be detected additionally (marked by “sLD”). These tiny droplets are approximately 50–150 nm in diameter. b Different kinds of droplets can be detected. (1) Many bigger LDs not labeled for PLIN2 (center). (2) Several small and midsize, PLIN2-positive, LDs are occasionally seen just approaching a non-labeled, larger LD for fusion (center). (3) Strongly PLIN2-labeled, newly endocytosed lipid-containing microvesicles, very small LDs (sLD). Many non-labeled, flask-like invaginations of plasma membrane, comprising clusters of vesicle-like structures (vls), can be detected in addition (Fig. 5b). Bars 500 nm
Specific oxidases in adipocytes and mammary gland
We next wanted to confirm the reported occurrence of XOR in adipocytes and tested commercially available abs for XOR, including abs for the homologous protein AOX (Fig. S5). In addition, we raised and characterized several new abs to XOR (Fig. 6a, Table S1). With further characterizations, it became evident that XOR is detectable solely with MLGM, whereas AOX is expressed in adipocytes and not with MLGM (Fig. S6). To confirm these results, we next performed mass spectrometric (MS) analysis and used Coomassie Blue–stained gel bands from MLGM material, from total cell lysate of preadipocytes and from total cell lysate of differentiated adipocytes (Fig. S7). We identified XOR with the MLGM reference sample and additional characteristic LD binding proteins: including BTN1A and PLIN2. In contrast, no XOR was detected in adipocytes; however again, in accordance with our immunoblotting results, the homologous enzyme AOX was identified instead, which has an almost identical molecular mass as XOR. Together with AOX, also PLIN4 and vimentin as known LD binding proteins were identified (Fig. S7).
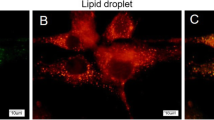
Biochemical characterization, IFM and IEM localization using human adipocytes and antibodies directed against XOR and AOX. (a) Immunoblotting of total cell lysates of adipocytes differentiated by AIM (lane P) and MLGM from human milk (lane M). Antibodies (abs) tested, respectively, (from left to right): mab Peri112.17 (PLIN1), pab AOX1-hCT-1323 (AOX), pab SOD2 (SOD2), pab hXO-CT-1318 (XO) and pab hXO-CT-1326 (XO*). The antibody specific for AOX reacts only with a 150-kD band of adipocytes. In contrast, at similar molecular weight position, abs for XOR react solely with MLGM material. Control abs PLIN1 and SOD2 react only within the lanes of adipocytes. (b, b′, b″) Density gradient centrifugation separations of AIM-stimulated preadipocytes, followed by immunoblot detection. Selected gradient fractions were tested with mab Peri112.17 (PLIN1) in (b), with pab AOX1-hCT-1323 (AOX) in (b′) and with pab SOD2 (SOD2) in (b″). Individual fraction numbers are given at top of (b). For gradient areas LD1 (top layer, fraction of low density), LD2 (intermediate density) and LD3 (cytosolic fraction, high density), compare our previous publications Heid et al. (2013) and Heid et al. (2014). Positions of molecular weight markers are given at the left margin. PLIN1, AOX and SOD2 are distributed essentially in different gradient areas, respectively. AOX is preferentially detected in the cytosolic fraction LD3, SOD2 in fraction LD2 and not, like PLIN1, in the LD fraction (top layer, LD1). (c) Double immunofluorescence microscopy showing PLIN1 (red) in comparison with AOX (green) staining. (d) Immunoelectron microscopy demonstrating labeling with AOX-specific antibody in the cytoplasm (lower part) and not in the LD region (upper part). No obvious co-localization of PLIN1 and AOX is seen in (c) and the cytoplasmic staining of AOX in (d) fits to the soluble, cytoplasmic occurrence of AOX (fraction LD3) detected in density gradient separation in (b′), whereas PLIN1 is detected LD bound (fraction LD1). Bars (c) 20 μm and (d) 1 μm
AOX association with LDs in adipocytes
AOX is found soluble in the cytoplasm during adipocyte conversion and LD expansion in adipocytes and not associated with LDs (Fig. 6b–d). With starvation condition, EM, density gradient separations and Western blotting, we demonstrate for the first time evidences for AOX-LD association and interaction in immediate vicinity to mitochondria (Fig. 7).
Immunoblot detection of AOX in differentially treated human adipocytes and electron micrograph of adipocytes subjected to fasting condition. a–c Density gradient centrifugation separations of preadipocytes stimulated by AIM for adipocyte conversion followed by immunoblots using polyclonal antibody specific for AOX (pab AOX-hCT-1323). a AIM-treated cells, b AIM-treated cells followed by additional supply of OA and c AIM-treated cells followed by a change of culture cell media for starvation and lipid depletion condition (Hank’s media). Individual fraction numbers of the gradient separation are given at the top (LD = top layer LD fraction; cp. Fig. 4 shown by Heid et al. 2014). Positions of molecular weight markers are given at the left margins. Positive reaction of fractions 22–28 (cytosolic fractions) can be detected with all treatments. AOX-positive reaction is seen in low and intermediate density LD fractions exclusively with Hank’s treatment (Fig. 8(c), arrows). d Electron micrograph of cells as used in Fig. 8c, showing an increase in number of mitochondria, many of them in the immediate vicinity and in very close contact with LDs (arrows). Bar 1 μm
Summarizing our new results obtained with adipocytes regarding association of PLINs and oxidases with LDs (Figs. 4 and 7) and comparing with data on lactating mammary gland cells (Mather and Keenan 1998) and cardiomyocytes (Wang et al. 2011) and from other sources, we recognized a basic and general tethering principle for LD connections to biomembranes (Table 1).
Cartoons of our results with a comparison of major LD-associated proteins detected in mammary gland and in adipocytes are presented in Fig. 8. Whereas PLIN2 and PLIN3 can be detected LD associated in non-lactating and in lactating epithelial cells as well as in mesenchymal preadipocytes and in differentiated adipocytes, PLIN4 is found only in preadipocytes and adipocytes. The transmembrane protein BTN is abundantly expressed only in lactating mammary gland, whereas PLIN1 is detected only in differentiated adipocytes. Oxidases found differ in their location: XOR is found in the mammary gland, AOX in adipocytes. These oxidases are mostly found within the cytoplasm of tissue and cultured cells. Yet XOR is also found LD bound at the inner apical plasma membrane and in secreted milk lipid globules and AOX becomes LD associated by fat depot reduction under starvation conditions in close proximity of mitochondria
Comparison of major LD-associated proteins and specific oxidases in human mammary gland tissue and adipocytes. LDs in non-lactating and lactating mammary gland as well as in non-differentiated and differentiated adipocytes are schematically presented (two boxes of the upper part). Sets of major LD-binding proteins found under these conditions are listed below (boxes in the middle part). PLIN2 and PLIN3 are expressed in all cases; PLIN4 is detectable in both non-differentiated and differentiated adipocytes, PLIN1 only in differentiated adipocytes. In lactating mammary gland, the milk-specific transmembrane proteins BTN1A and XOR are major LD-associated proteins. In boxes of the lower part of the figure, specific oxidases found with our investigations are summarized, respectively. XOR was expressed strongly in mammary gland. However in contrast to Cheung et al. (2007), XOR could not be confirmed in adipocytes. AOX was found instead. These two different enzymes are localized mainly soluble in the cytoplasm and only seen LD associated in certain situations: (1) XOR at the apical plasma membrane (PM) during milk secretion and at secreted mammary gland milk LDs and (2) AOX in adipocytes under starvation and nutrition depletion conditions at LDs in the neighborhood and contact to mitochondria
Discovery of a complementary localization of XOR and AOX
Finally, so far unknown, when summarizing and comparing former results published on the immunolocalization of XOR with immunohistochemical localization data published for AOX, we discovered an obviously mutually exclusive and complementary localization of these two oxidases in diverse cell types (Table 2). Only capillaries of adipose tissue and not the adipocytes itself express XOR but AOX was found to be present and expressed on the protein level in adipocytes.
Discussion
The report on XOR in adipose tissue and adipocytes and the postulated involvement of this enzyme in adipogenesis (Cheung et al. 2007) gave rise to revisit and extend our former investigations on the formation of LDs. We found strikingly similar sets of major LD binding proteins reported for MLGs from the lactating mammary gland and for adipocytes. XOR is tethering cytoplasmic LDs in the lactating mammary gland to the apical plasma membrane by interacting with the LD binding protein PLIN2 and the cytoplasmic part of the transmembrane, milk specific protein BTN (Mather and Keenan 1998; McManaman et al. 2002; Heid and Keenan 2005; Monks et al. 2016). We wondered what specific role such an enzyme might play in adipocytes, cells that are LD storage cells and not LD secretory cells.
We highlight our major findings
-
1.
EM and IEM on the formation of LDs in adipocytes are extended and connections of LDs with mitochondria under starvation conditions are presented.
-
2.
We generated novel and reliable abs for XOR and AOX suitable for new experiments.
-
3.
We identified AOX expressed in adipocytes instead of XOR as previously reported.
-
4.
We recognized an exclusive and complementary localization of XOR and AOX in diverse cell types and summarized all the data in tables and cartoons.
-
5.
A hypothesis is presented where XOR together with PLIN2 is tethering LDs to the apical plasma membrane of lactating mammary gland cells and AOX together with PLIN1 is linking LDs with mitochondria in adipocytes under starvation condition.
Is XOR involved in adipogenesis?
XOR has been suggested as a novel regulator of adipogenesis (Cheung et al. 2007); however in this report, XOR was not shown at the protein expression level and the XOR-related enzyme AOX was not tested. Like XOR, AOX belongs to a family of molybdo-flavoproteins with an identity towards XOR of 49.7% on the protein level and with almost a similar molecular mass of 150 kD. The genes encoded have almost identical intron/exon organization and both enzymes have a similar spectrum of enzymatic reactions. Therefore, the oxidase activity of XOR activity measurement in the absence and presence of NAD+, claimed to distinguish activities between XO and XHD, could be prompted also by AOX and oxygen. Current data are based on microarray analysis, activity measurement, a HPLC assay for uric acid levels, working with PPARy agonists, which all can be influenced also by AOX, because both enzymes, XOR and AOX, are able to generate ROS (Kundu et al. 2007; Kundu et al. 2012). In addition, inhibitors like allopurinol and others, used for generating these data, can work on both enzymes and can create misinterpretations (see, e.g., Weidert et al. 2014; Williams et al. 2014). The evolution of AOX from XOR was by gene duplication and the enzymes are vicinally situated on the same chromosome (see, e.g., Krenitsky 1978). Therefore, the claimed cascade of factors that controls adipogenesis might hold true perhaps also for AOX and not exclusively for XOR. To date, many researchers believe that XOR is the principal enzyme responsible for oxidation of purine metabolites to uric acid and that hyperuricemia is highly correlated with increased adiposity, obesity and the metabolic syndrome (Berry and Hare 2004; Harrison 2002; Pacher et al. 2006). Still, the question remains: why could XOR not be shown directly at the protein level in adipocytes? Interestingly, Weigert et al. reported on AOX in high amounts in adipose tissue and mouse 3T3-L1 and demonstrated with fenofibrate, an agonist of PPAR-α, reduced AOX protein contents in differentiated 3T3-L1 cells (Weigert et al. 2008).
These different interpretations prompted us to revisit data on adipogenesis with special emphasis on these oxidases XOR and AOX.
Endogenously and exogenously derived LDs in adipocytes and validation of novel antibodies to XOR and AOX
Human preadipocytes were converted to adipocytes to test commercially available abs for XOR. We wanted to confirm the expression of XOR during adipogenesis in relation to the LD binding perlipins. We took an approach on LD formation and generated two different kinds of LDs (Fig. 1b; described by Heid et al. 2014). We obtained in cells internally generated, endogenous LDs derived from ER with monolayer membranes (Murphy and Vance 1999; Heid and Keenan 2005; Martin and Parton 2006; Wilfling et al. 2013; Choudhary et al. 2015). By addition of albumin-complexed OA to the culture medium, exogenous LDs with bilayer membranes from the uptake via an endocytosis process were additionally generated. By IFM, the huge variety and heterogeneity of resulting LDs by such approaches could be demonstrated (Fig. S1). We discovered using proper abs and by mass spectrometry that instead of the proclaimed XOR, the related enzyme AOX is expressed in adipocytes (see below).
EM findings on vimentin, layers of ER and the formation of LDs
In a next step, we extended our previous findings on the perilipin-vimentin cortex surrounding nascent LDs (Figs. 2,S2 and S4; see Heid et al. 2014). These structures of forming LDs are often encased by multiple layers of ER. With starting the conversion condition, occasionally some mitochondria seemed to be linked via vimentin intermediate filaments to LDs (Fig. S2b). In general, only a few, small mitochondria are seen in the cytoplasm during LD forming and the perilipin-vimentin cortex and ER layers are keeping the growing LDs separated from mitochondria (Figs. 2 and S3). We would like to emphasize that we and formerly others (Novikoff et al. 1980; Franke et al. 1987; Heid et al. 2014), so far, have not found by EM any evidence yet for LDs contacting microtubules. In support of our findings are results on the ablation of vimentin in steroidogenic tissues where the movement of cholesterol from cytosolic LDs to mitochondria is defected (Shen et al. 2012).
EM and OA uptake
When albumin-complexed OA was added to the conversion medium of adipocytes (cp. Fig. 1b), almost immediately many endocytotic vesicles and invaginations appear in the cytoplasm, especially near the plasma membrane (Fig. 3a). These endocytotic vesicles have bilayer membranes derived from the plasma membrane material, also seen in clusters of “vesicles-like structures” or of “rosette-like structures” (Fig. 3b). These structures were first described in rat adipose tissue (Williamson 1964) and in mouse 3T3-L1 cultured cells (Novikoff et al. 1980). The invaginations of the plasma membrane are not labeled by perilipin abs. Still in the process of endocytosis, these invaginations are likely be filled with OA cargo. As further consequence of OA supply and “ER decay,” the tight intermediate filament structures at the surface of LDs begin to loosen and occasionally, via these vimentin intermediate filaments, mitochondria can be seen linked to individual LDs (Fig. 3c). How bilayer membrane-surrounded, endocytosis-derived LDs can assemble and fuse with monolayer membrane-surrounded, ER-derived LDs, is still a matter of debate. One explanation might be the unique architecture of the “monolayer LDs”, which can give rise to “lipidic bridges” (Schuldiner and Bohnert 2017).
IEM localization of perilipins in adipocytes
IEM localization of PLIN1 upon adipocyte conversion is solely found at the surface of endogenously, ER-derived LDs (Fig. 4). These LDs are connected by a network of intermediate filaments. With OA uptake, smaller vesicle-like structures appear in the sections; many are seen in clusters. These are not labeled for PLIN1 but are cross-sectioned invaginations. In addition, possible association sites of mitochondria and LDs with positive labeling of PLIN1 can be detected (Fig. 4, black brackets). These IEM results support an involvement of PLIN1 in tethering mitochondria with LDs (see below for further arguments). IEM localization in adipocytes of PLIN2 is shown in Fig. 5. PLIN2-positive labeling of LDs is seen as a minor population of midsize droplets during adipocyte conversion in midst of PLIN1-positive, bigger LDs. LD anchored by intermediate filaments is clearly detectable. Clusters of very small, positively labeled droplets can be detected additionally (“sLD”). These seem to be derived from endocytosed lipid material by OA treatment.
AOX is expressed in adipocytes and links LDs and mitochondria under nutrition depletion
Despite testing a panel of abs against XOR with samples of adipocytes, we were not able to confirm the report on XOR on the protein level (Fig. S5). Additional experiments and having identified unambiguously AOX instead of XOR in adipocytes by mass spectrometry (MS) and immunoblotting using a broad spectrum of commercial available and newly generated, validated specific abs (Figs. 6, S6, and S7; Table S1), we wondered why the XOR related enzyme AOX so far was not tested but rather excluded in the work of many reports. So far, only one report describes AOX in high amounts in fat depots and mouse 3T3-L1 cells (Weigert et al. 2008). AOX is known for broad substrate specification used in many clinical studies on the metabolism of drugs and xenobiotics (Terao et al. 2016; Romao et al. 2017; Beedham 2019). Humans are characterized by a single AOX enzyme, while rodents express four isoenzymes (AOX1–4). Both enzymes AOX and XOR are able to generate besides ROS also nitric oxide (NO) (see Maia and Moura 2018; Bender and Schwarz 2018). Under normal culturing conditions of adipocytes, AOX localizes in the cytoplasm of cells (Fig. 6b–d). XOR is also localized soluble in epithelial cells of the mammary gland (Bruder et al. 1984). In lactating mammary gland epithelial cells, XOR is binding additionally the LD protein PLIN2 and supporting with LDs docking to the apical plasma membrane via BTN. XOR is clustering BTN within the apical membrane and regulates milk lipid secretion into milk ducts (Mather and Keenan 1998; Jeong et al. 2009). This clustering was shown also with mammary specific XOR knockout mice, where XOR was found to make the secretion of LDs highly efficient (Monks et al. 2016). Knowing that a distinct combination of perilipins and molybdo-flavoenzymes—PLIN2 and XOR—is responsible for delivering/transporting LDs to the apical plasma membrane in the lactating mammary gland, we speculate that such a similar tethering system might also exist within adipocytes under starvation condition (Fig. 7). LD trafficking to mitochondria in starved mouse embryonic fibroblasts cells (MEFs) was described recently (Rambold et al. 2015) and thus, we propose for adipocytes a combination of PLIN1 and AOX as a tether for such linkages (Figs. 4 and 7). Adipose triglyceride lipase (ATGL) might also be involved for direct, immediate release of fatty acids (FAs) at the LD-mitochondria interface (Smirnova et al. 2006; Granneman and Moore 2008; Haemmerle et al. 2011; Walch et al. 2015). This assumption awaits verification but some arguments in favor are given by a report with experiments using Saccharomyces cerevisiae (see below; Pu et al. 2011). Direct delivery of FAs from the storage organelle to the degradation/β-oxidation organelle would be a highly efficient way to prevent otherwise cytotoxic FA effects in the cytosol. Whether acyl-CoA synthetases (ACSLs) and carnitine palmitoyltransferases (CPTs) (Young et al. 2018; Coleman 2019) play a role as a docking station of outer mitochondria membranes (OMMs) remains to be established. The same holds true also for synaptosomal-associated protein 23 (SNAP23) reported for interaction of LDs and mitochondria (Jagerstrom et al. 2009; Strauss et al. 2016).
LD binding protein PLIN5 of muscle cells is a major player in recruiting LDs to mitochondria (Wang et al. 2011; Andersson et al. 2017; Pribasnig et al. 2018; Gemmink et al. 2018). AOX might be the possible oxidase partner participating together with PLIN5 in linking of LDs to mitochondria in myocytes, because XOR is localized only in capillary endothelial cells of heart and muscle tissue (Jarasch et al. 1981; Jarasch et al. 1986) and AOX is found during myogenesis in the mouse skeletal myoblast cell line C2C12 (Kamli et al. 2014). A summary of the combinations for perilipins and oxidases tethering LDs to specific membranes is given in Table 1.
Localization of XOR and AOX in cells and tissues
A surprising finding of our study is the recognition of a complimentary, mutually exclusive localization of the molybdo-flavoenzymes XOR and AOX in diverse cell types (Table 2). XOR expression has so far only been reported on the protein level solely in epithelial cells of lactating mammary gland and in capillary endothelial cells of small blood vessels (Jarasch et al. 1981; Jarasch et al. 1986; Bruder et al. 1984). For clinical aspects of endothelial XOR in reperfusion injury and inflammatory signal transduction, see Meneshian and Bulkley (2002). For therapeutic effects of XOR inhibitors in a comprehensive compilation of clinical studies, see Pacher et al. (2006). The many discrepancies currently found in the literature for XOR originate mainly from incorrect addressing and clear discrimination between the two enzymes XOR and AOX. With the knowledge of source and localization of the two enzymes by using proper antibodies, the measurements on purine metabolism—analyzing hypoxanthine and uric acid in blood and urine—have to be credited in most cases rather to AOX than to XOR.
Interestingly, Williams et al. (2014) requested that hundreds of studies using high doses of allopurinol claimed as XO inhibitors should be revisited. Of course, these corrections should be extended also for the localization and antibody-based publications on XOR and AOX. Starting with publicly available reports on the localization of the enzymes (Jarasch et al. 1986; Moriwaki et al. 2001; Weigert et al. 2008), we compared both enzymes (Table 2). Our results now highlight that XOR is not an ubiquitously and constitutively expressed metallo-flavoprotein as stated in most current XOR publications.
Other functions than housekeeping
XOR and AOX; both enzymes can activate lipid peroxidation. This process generates naturally ROS on a small level in the body. Thereby, polyunsaturated FAs of membranes are attacked and a chain reaction is started. When the antioxidant defense mechanism is overcome, great damage and destruction of membrane lipids could take place. ROS production can change membrane properties and alter membrane deformability.
XOR has been described as a housekeeping protein with enzymatic functions in purine catabolism and additionally in a variety of other roles. The enzyme has a protective and antimicrobial defense mechanism, can participate in wound healing through effects on ROS production and is supposed to be part of an innate immune system (Jarasch et al. 1986; Vorbach et al. 2002; Vorbach et al. 2003; Vorbach et al. 2006; Madigan et al. 2015). Mouse XOR gene knockout (Vorbach et al. 2002; Ohtsubo et al. 2004; Ohtsubo et al. 2009; Murakami et al. 2014) and mouse isoenzyme AOX4 knockout studies are beyond the scope of this manuscript and have to be discussed separately. Besides ROS, the generation of NO by the two enzymes, XOR and AOX, should be emphasized (Lundberg et al. 2011; Millar et al. 1998). With its localization in capillary endothelial cells, XOR is a major player and in a key position to control vasodilatation of small vessels by NO and inflammation events by ROS. Blood pressure lowering can also be achieved by allopurinol, an inhibitor of both enzymes, XOR and AOX. This drug is mostly prescribed in gout disease for the reduction of uric acid formation. Using a large clinical data collection of adults with hypertension, allopurinol-treated patients were associated with a significantly lower risk of both stroke and cardiac events than with non-exposed patients (MacIsaac et al. 2016).
In addition to these multiple activities and roles of XOR and AOX, we propose completely new functions, tethering of LDs to biomembranes in conjunction with specific perlipins (Table 1).
Pu et al. (2011) investigated the interaction between LDs and mitochondria in S. cerevisiae. Using a bimolecular fluorescence complementation assay, they identified an LD protein named Erg6 that can link to mitochondria. Erg6 is described as a regulator of membrane permeability and fluidity (Gaber et al. 1989). With additional Erg6 binding assays, Pu and coworkers came up with other associated LD proteins. Identified were proteins with sterol esterase activity (Yeh1), with triglyceride lipase activity (Tgl3, Tgl4) and with oxidoreductase activity (Yor246c). With lipases and the oxidoreductase binding on LDs and Erg6 linking to mitochondria, they find—at least viewed from functional aspects—a very similar set of proteins in S. cerevisiae as we now propose for human adipocytes. Since early in evolution an oxidoreductase enzyme seems to be important for docking and locally changing membrane properties for proper channeling of lipid products.
Abbreviations
- abs:
-
Antibodies
- AOX:
-
Aldehyde oxidase
- ATGL:
-
Adipose triglyceride lipase
- BTN or BTN1a:
-
Butyrophilin
- EM:
-
Electron microscopy
- ER:
-
Endoplasmatic reticulum
- FA:
-
Fatty acids
- IEM:
-
Immunoelectron microscopy
- IFM:
-
Immunofluorescence microscopy
- LD:
-
Lipid droplet
- MLGM:
-
Milk lipid globule membrane
- MS:
-
Mass spectrometry
- NO:
-
Nitric oxide
- OA:
-
Oleic acid
- OMM:
-
Outer mitochondrial membrane
- PLIN:
-
Perilipin
- PPAR:
-
Peroxisome proliferator-activated receptor
- ROS:
-
Reactive oxygen species
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- sLD:
-
Small lipid droplet
- VIM:
-
Vimentin
- XDH:
-
Xanthine dehydrogenase
- XO:
-
Xanthine oxidase
- XOR:
-
Xanthine oxidoreductase
References
Andersson L, Drevinge C, Mardani I, Dalen KT, Stahlman M, Klevstig M, Lundqvist A, Haugen F, Adiels M, Fogelstrand P, Asin-Cayuela J, Hulten LM, Levin M, Ehrenborg E, Lee YK, Kimmel AR, Boren J, Levin MC (2017) Deficiency in perilipin 5 reduces mitochondrial function and membrane depolarization in mouse hearts. Int J Biochem Cell Biol 91:9–13
Baker M (2015) Reproducibility crisis: blame it on the antibodies. Nature 521:274–276
Battelli MG, Bortolotti M, Polito L, Bolognesi A (2019) Metabolic syndrome and cancer risk: the role of xanthine oxidoreductase. Redox Biol 21:101070
Beedham C (2019) Aldehyde oxidase; new approaches to old problems. Xenobiotica 1–17. https://doi.org/10.1080/00498254.2019.1626029
Bender D, Schwarz G (2018) Nitrite-dependent nitric oxide synthesis by molybdenum enzymes. FEBS Lett 592:2126–2139
Berry CE, Hare JM (2004) Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 555:589–606
Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C (1995) Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 36:1211–1226
Bruder G, Jarasch ED, Heid HW (1984) High concentrations of antibodies to xanthine oxidase in human and animal sera. Molecular characterization. J Clin Invest 74:783–794
Cheung KJ, Tzameli I, Pissios P, Rovira I, Gavrilova O, Ohtsubo T, Chen Z, Finkel T, Flier JS, Friedman JM (2007) Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab 5:115–128
Choudhary V, Ojha N, Golden A, Prinz WA (2015) A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J Cell Biol 211:261–271
Coleman RA (2019) It takes a village: channeling fatty acid metabolism and triacylglycerol formation via protein interactomes. J Lipid Res 60:490–497
Franke WW, Krien S, Brown RM Jr (1969) Simultaneous glutaraldehyde-osmium tetroxide fixation with postosmication. An improved fixation procedure for electron microscopy of plant and animal cells. Histochemie 19:162–164
Franke WW, Heid HW, Grund C, Winter S, Freudenstein C, Schmid E, Jarasch ED, Keenan TW (1981) Antibodies to the major insoluble milk fat globule membrane-associated protein: specific location in apical regions of lactating epithelial cells. J Cell Biol 89:485–494
Franke WW, Hergt M, Grund C (1987) Rearrangement of the vimentin cytoskeleton during adipose conversion: formation of an intermediate filament cage around lipid globules. Cell 49:131–141
Gaber RF, Copple DM, Kennedy BK, Vidal M, Bard M (1989) The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol 9:3447–3456
Gemmink A, Daemen S, Kuijpers HJH, Schaart G, Duimel H, Lopez-Iglesias C, van Zandvoort M, Knoops K, Hesselink MKC (2018) Super-resolution microscopy localizes perilipin 5 at lipid droplet-mitochondria interaction sites and at lipid droplets juxtaposing to perilipin 2. Biochim Biophys Acta Mol Cell Biol Lipids 1863:1423–1432
Granneman JG, Moore HP (2008) Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab 19:3–9
Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C (1991) Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266:11341–11346
Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rulicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R (2011) ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med 17:1076–1085
Harrison R (2002) Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 33:774–797
Heid HW, Keenan TW (2005) Intracellular origin and secretion of milk fat globules. Eur J Cell Biol 84:245–258
Heid H, Rickelt S, Zimbelmann R, Winter S, Schumacher H, Dorflinger Y (2013) Lipid droplets, perilipins and cytokeratins--unravelled liaisons in epithelium-derived cells. PLoS One 8:e63061
Heid H, Rickelt S, Zimbelmann R, Winter S, Schumacher H, Dorflinger Y, Kuhn C, Franke WW (2014) On the formation of lipid droplets in human adipocytes: the organization of the perilipin-vimentin cortex. PLoS One 9:e90386
Jagerstrom S, Polesie S, Wickstrom Y, Johansson BR, Schroder HD, Hojlund K, Bostrom P (2009) Lipid droplets interact with mitochondria using SNAP23. Cell Biol Int 33:934–940
Jarasch ED, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW (1981) Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell 25:67–82
Jarasch ED, Bruder G, Heid HW (1986) Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl 548:39–46
Jeong J, Rao AU, Xu J, Ogg SL, Hathout Y, Fenselau C, Mather IH (2009) The PRY/SPRY/B30.2 domain of butyrophilin 1A1 (BTN1A1) binds to xanthine oxidoreductase: implications for the function of BTN1A1 in the mammary gland and other tissues. J Biol Chem 284:22444–22456
Kamli MR, Kim J, Pokharel S, Jan AT, Lee EJ, Choi I (2014) Expressional studies of the aldehyde oxidase (AOX1) gene during myogenic differentiation in C2C12 cells. Biochem Biophys Res Commun 450:1291–1296
Krenitsky TA (1978) Aldehyde oxidase and xanthine oxidase--functional and evolutionary relationships. Biochem Pharmacol 27:2763–2764
Kundu TK, Hille R, Velayutham M, Zweier JL (2007) Characterization of superoxide production from aldehyde oxidase: an important source of oxidants in biological tissues. Arch Biochem Biophys 460:113–121
Kundu TK, Velayutham M, Zweier JL (2012) Aldehyde oxidase functions as a superoxide generating NADH oxidase: an important redox regulated pathway of cellular oxygen radical formation. Biochemistry 51:2930–2939
Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E (2011) Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res 89:525–532
MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, Touyz RM, Dawson J (2016) Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension 67:535–540
Madigan MC, McEnaney RM, Shukla AJ, Hong G, Kelley EE, Tarpey MM, Gladwin M, Zuckerbraun BS, Tzeng E (2015) Xanthine oxidoreductase function contributes to normal wound healing. Mol Med 21:313–322
Maia LB, Moura JJG (2018) Putting xanthine oxidoreductase and aldehyde oxidase on the NO metabolism map: nitrite reduction by molybdoenzymes. Redox Biol 19:274–289
Martin S, Parton RG (2006) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378
Martin HM, Hancock JT, Salisbury V, Harrison R (2004) Role of xanthine oxidoreductase as an antimicrobial agent. Infect Immun 72:4933–4939
Mather IH, Keenan TW (1998) Origin and secretion of milk lipids. J Mammary Gland Biol Neoplasia 3:259–273
McCord JM, Roy RS, Schaffer SW (1985) Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv Myocardiol 5:183–189
McManaman JL, Palmer CA, Wright RM, Neville MC (2002) Functional regulation of xanthine oxidoreductase expression and localization in the mouse mammary gland: evidence of a role in lipid secretion. J Physiol 545:567–579
Meneshian A, Bulkley GB (2002) The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 9:161–175
Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR (1998) Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett 427:225–228
Monks J, Dzieciatkowska M, Bales ES, Orlicky DJ, Wright RM, McManaman JL (2016) Xanthine oxidoreductase mediates membrane docking of milk-fat droplets but is not essential for apocrine lipid secretion. J Physiol 594:5899–5921
Moriwaki Y, Yamamoto T, Takahashi S, Tsutsumi Z, Hada T (2001) Widespread cellular distribution of aldehyde oxidase in human tissues found by immunohistochemistry staining. Histol Histopathol 16:745–753
Murakami N, Ohtsubo T, Kansui Y, Goto K, Noguchi H, Haga Y, Nakabeppu Y, Matsumura K, Kitazono T (2014) Mice heterozygous for the xanthine oxidoreductase gene facilitate lipid accumulation in adipocytes. Arterioscler Thromb Vasc Biol 34:44–51
Murphy DJ, Vance J (1999) Mechanisms of lipid-body formation. Trends Biochem Sci 24:109–115
Novikoff AB, Novikoff PM, Rosen OM, Rubin CS (1980) Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol 87:180–196
Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T (2004) Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ Res 95:1118–1124
Ohtsubo T, Matsumura K, Sakagami K, Fujii K, Tsuruya K, Noguchi H, Rovira II, Finkel T, Iida M (2009) Xanthine oxidoreductase depletion induces renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules. Hypertension 54:868–876
Pacher P, Nivorozhkin A, Szabo C (2006) Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58:87–114
Pribasnig M, Kien B, Pusch L, Haemmerle G, Zimmermann R, Wolinski H (2018) Extended-resolution imaging of the interaction of lipid droplets and mitochondria. Biochim Biophys Acta Mol Cell Biol Lipids 1863:1285–1296
Pu J, Ha CW, Zhang S, Jung JP, Huh WK, Liu P (2011) Interactomic study on interaction between lipid droplets and mitochondria. Protein Cell 2:487–496
Rambold AS, Cohen S, Lippincott-Schwartz J (2015) Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 32:678–692
Romao MJ, Coelho C, Santos-Silva T, Foti A, Terao M, Garattini E, Leimkuhler S (2017) Structural basis for the role of mammalian aldehyde oxidases in the metabolism of drugs and xenobiotics. Curr Opin Chem Biol 37:39–47
Schuldiner M, Bohnert M (2017) A different kind of love—lipid droplet contact sites. Biochim Biophys Acta Mol Cell Biol Lipids 1862:1188–1196
Shen WJ, Zaidi SK, Patel S, Cortez Y, Ueno M, Azhar R, Azhar S, Kraemer FB (2012) Ablation of vimentin results in defective steroidogenesis. Endocrinology 153:3249–3257
Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL (2006) ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep 7:106–113
Strauss JA, Shaw CS, Bradley H, Wilson OJ, Dorval T, Pilling J, Wagenmakers AJ (2016) Immunofluorescence microscopy of SNAP23 in human skeletal muscle reveals colocalization with plasma membrane, lipid droplets, and mitochondria. Physiol Rep 4(1):e12662. https://doi.org/10.14814/phy2.12662
Sztalryd C, Kimmel AR (2014) Perilipins: lipid droplet coat proteins adapted for tissue-specific energy storage and utilization, and lipid cytoprotection. Biochimie 96:96–101
Terao M, Romao MJ, Leimkuhler S, Bolis M, Fratelli M, Coelho C, Santos-Silva T, Garattini E (2016) Structure and function of mammalian aldehyde oxidases. Arch Toxicol 90:753–780
Vorbach C, Scriven A, Capecchi MR (2002) The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev 16:3223–3235
Vorbach C, Harrison R, Capecchi MR (2003) Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol 24:512–517
Vorbach C, Capecchi MR, Penninger JM (2006) Evolution of the mammary gland from the innate immune system? Bioessays 28:606–616
Walch L, Copic A, Jackson CL (2015) Fatty acid metabolism meets organelle dynamics. Dev Cell 32:657–658
Wang H, Sztalryd C (2011) Oxidative tissue: perilipin 5 links storage with the furnace. Trends Endocrinol Metab 22:197–203
Wang H, Sreenivasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong DW, Stanley WC, Sztalryd C (2011) Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res 52:2159–2168
Weidert ER, Schoenborn SO, Cantu-Medellin N, Choughule KV, Jones JP, Kelley EE (2014) Inhibition of xanthine oxidase by the aldehyde oxidase inhibitor raloxifene: implications for identifying molybdopterin nitrite reductases. Nitric Oxide 37:41–45
Weigert J, Neumeier M, Bauer S, Mages W, Schnitzbauer AA, Obed A, Groschl B, Hartmann A, Schaffler A, Aslanidis C, Scholmerich J, Buechler C (2008) Small-interference RNA-mediated knock-down of aldehyde oxidase 1 in 3T3-L1 cells impairs adipogenesis and adiponectin release. FEBS Lett 582:2965–2972
Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, Liu X, Buhman KK, Coleman RA, Bewersdorf J, Farese RV Jr, Walther TC (2013) Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell 24:384–399
Williams CD, McGill MR, Lebofsky M, Bajt ML, Jaeschke H (2014) Protection against acetaminophen-induced liver injury by allopurinol is dependent on aldehyde oxidase-mediated liver preconditioning. Toxicol Appl Pharmacol 274:417–424
Williamson JR (1964) Adipose tissue morphological changes associated with lipid mobilization. J Cell Biol 20:57–74
Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE (2005) S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem 280:19146–19155
Yisireyili M, Hayashi M, Wu H, Uchida Y, Yamamoto K, Kikuchi R, Shoaib Hamrah M, Nakayama T, Wu Cheng X, Matsushita T, Nakamura S, Niwa T, Murohara T, Takeshita K (2017) Xanthine oxidase inhibition by febuxostat attenuates stress-induced hyperuricemia, glucose dysmetabolism, and prothrombotic state in mice. Sci Rep 7:1266
Young PA, Senkal CE, Suchanek AL, Grevengoed TJ, Lin DD, Zhao L, Crunk AE, Klett EL, Fullekrug J, Obeid LM, Coleman RA (2018) Long-chain acyl-CoA synthetase 1 interacts with key proteins that activate and direct fatty acids into niche hepatic pathways. J Biol Chem 293:16724–16740
Acknowledgments
We greatly appreciate Werner W. Franke and Thomas W. Keenan for their continuous generous support and advice for more than 40 years. We especially thank Stefanie Winter, Caecilia Kuhn, Heiderose Schumacher and Edeltraut Noffz (Helmholtz Group for Cell Biology, DKFZ) for valuable help with biochemical characterizations and cell culture work. Special acknowledgement goes to Andrea Janicová (Department Biology, Friedrich-Alexander University of Erlangen-Nuremberg, Germany) for experiments in the framework of her Master Thesis at the Helmholtz Group for Cell Biology in Heidelberg. Martina Schnoelzer and Uwe Warnken (Functional Proteome Analysis, DKFZ) are gratefully acknowledged for proteomic analysis. We thank Ian Mather (University of Maryland, College Park, MD, USA) for his supply of one XOR antibody and Nicole B. (Heidelberg) for her generous supply of milk used for the isolation of milk lipid globule membranes.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: HH. Performed the experiments: HH, RZ, YD and SR. Analyzed the data: HH and SR. Wrote the paper: HH and SR. Drawings and finalizing figures: HH and RZ.
Corresponding author
Additional information
This work is dedicated to Werner W. Franke on the occasion of his 80th birthday.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Table S1: Antibodies.
Novel antibodies (abs) generated and commercially available antibodies used in this study were directed against: (1) LD-binding proteins perilipin 1-4 (PLIN1-4), (2) vimentin, (3) butyrophilin (BTN1A), (4) xanthine oxidoreductase (XOR), (5) aldehyde oxidase (AOX) and (6) superoxide dismutase 2 (SOD2). Ab designation, animal source used for ab generation, proteins or peptides used for immunization - including data on amino acid (aa) positions of antigens within the target protein sequence, commercial information of suppliers, corresponding catalog numbers, are given. Examples of testing and evaluation of the abs listed are shown in Figs. 7a, S5, S6. We noticed that several abs (stated to be specific for XOR and AOX) did not react as expected in our abs evalution and characterization. Some abs react also with other/additional, known and unknown protein bands, and some did not react at all. E.g. XOR ab EPR4605 reacts not only with XOR but also cross-reacts with AOX and with several protein bands of the reference lane (see also immunoblot data in Fig. S5d–e). No antigen information is supplied with this ab. Therefore we raised novel abs in addition, marked useful abs for XOR and AOX in Table S1 (asterisk) (PDF 528 kb).
Figure S1: Laser scanning microscopy showing endogenously and exogenously derived LDs in human adipocyte cells labelled with perilipin-specific antibodies.
(a–d) Double staining using abs for PLIN1 (a–d; red) versus PLIN2 (a–b; green) or PLIN3 (c–d; green). Early stages of differentiating adipocytes, were additionally treated with OA, leading to a heterogeneity of LDs of various sizes and specific staining. (a) Adipocyte induction media (AIM)-treatment for 3 days gives rise to endogenously ER-derived LDs positive for PLIN1 (red). OA-treatment and endocytotic uptake leads to many additional LDs positive for PLIN2 (green). Co-localization of droplet-staining – caused by LD-fusion events - is seen in yellow. (b) Enlarged motif with similar staining as presented in (a), showing the huge variety of LDs. Many examples of mosaic-like, patchy red-green and yellow-stained surfaces of LD can be seen. (c) Of note, even short OA-treatment (20 min) leads to increase of endocytosis and many new, very small PLIN3-positive droplets (green). Occasionally some of these LDs adhere to the much bigger PLIN1-positive droplets (red). (d) Longer OA-treatment (120 min) leads to larger-sized LDs positive for PLIN3 (green) and overall to a higher number of merged PLIN1/PLIN3 droplets (yellow). Nuclear staining was with DAPI (blue). Bars: 20 µm (PNG 550 kb)
Figure S2: Electron micrographs showing LDs of human preadipocytes upon brief stimulation for adipocyte differentiation, revealing association of vimentin intermediate filaments and special arrangement of endoplasmatic reticulum (ER) structures.
(a) Survey micrograph with emergence of two very small droplets (sLDs) surrounded and encased by intermediate filament bundles of the “wide-spacing type” (for definition see Heid et al. 2014). These LDs are found in midst of bigger, differentially more advanced LDs. Some mitochondria (M) are loosely connected to LDs by intermediate filaments at this stage of differentiation. (b) Beginning of the assembly of smooth ER tubules and cisternae (brown-pseudo-colored) near LDs. In grazing sections, a dense network of intermediate filaments is seen directly at the LD surface (“high density spacing”, open arrows; cross-sectioned as regular and periodical arrays of dots, black bold arrows). Bars: 500 nm (PNG 1.09 mb)
Figure S3: Electron micrographs showing LDs of human preadipocytes after stimulation for adipocyte differentiation with characteristic intermediate filament and ER structures in advanced differentiation stages.
(a) Arrays of “high density spacing” vimentin bundles are forming net- and cage-like structures in cross-sections, directly at the surface of growing droplets (arrows). These LD-intermediate filament structures are encased almost completely by at least one layer of smooth ER. (b) Differentiation stage with increasing numbers of ER layers surrounding LDs. Regular arrays of anchored intermediate filaments (arrows). Note: Intermediate filaments and ER are engulfing LDs. Occasionally entrapped mitochondria (M) are not located and connected directly to LDs but somewhat excluded by ER layers. Bars: (a) 500 nm; (a) 200 nm (PNG 851 kb)
Figure S4: Electron micrographs showing LDs in human preadipocytes after stimulation for adipocyte differentiation, showing specific situations of ER layers surrounding LDs.
(a) Group of LDs surrounded by layers of ER cisternae. (b) Micrograph showing two normal developing LDs with characteristic multiple ER layers (left side) and as an exceptional case, one smaller droplet bordering directly to mitochondria (M) inside multiple ER layers (right side). Bars: 500 nm (PNG 912 kb)
Figure S5: Examples of immunoblotting experiments using human adipocyte cell lysates and human milk lipid globule membrane (MLGM) for testing XOR and AOX antibodies.
(a) Reference proteins, total cell lysates of predadipocytes (PO), adipocytes differentiated by AIM (P) and MLGM (M) were separated by SDS-PAGE, transferred to PVDF membrane and Coomassie blue-stained. Positions of molecular weight markers are given on the left margin of (a). (b–e) Parallel separations as shown in (a) were used for testing various commercially available antibodies (for antibodies see Table S1). (b) pab XDH N-term (XOR, Abgent); (c) mab AOX1 (D-8) (AOX, Santa Cruz); (d) mab Xanthine Oxidase (EPR4605; XOR, Abcam); (e) Merge of pictures shown in a and d. Note: Whereas the antibody for XOR from Abgent reacts specifically with a 150kD band of MLGM (and a known breakdown product at 60kD) and the antibody for AOX (here used as control antibody, specific for the XOR-related, homologous enzyme AOX) from Santa Cruz specifically at 150kD with both used adipocyte lanes, the XOR antibody from Abcam cross-reacts with AOX in adipocytes and several other bands, including several strongly reacting bands of the protein marker lane (PNG 205 kb)
Figure S6: Expression of major LD-binding proteins, XOR and AOX in human adipocytes and human MLGM revealed by immunoblotting.
(a) Total cell lysates of predadipocytes (lane PO), preadipocytes stimulated by AIM and OA (lane P) and MLGM (lane M) were separated by SDS-PAGE, transferred to PVDF membrane and Coomassie blue-stained. (b–h) Membranes were used to test: (b) pab XDH N-term (XOR) (c) mab Butyrophilin 102.43 (BTN1A) (d) pab Adipo-hCT423 (PLIN2) (e) mab TIP47.49.19 (PLIN3) (f) mab Peri112.17 (PLIN1) (g) pab S3-12-hNT-1 (PLIN4) (h) mab AOX1(D-8) (AOX) (see also Table S1). Positions of molecular weight markers are given on the left margin of (a). Note: XOR and BTN1A are MLGM-specific. PLIN2 and PLIN3 can be detected in MLGM and in adiopocytes. PLIN1, PLIN4 and AOX are found only and specifically in adipocyte material (PNG 187 kb)
Figure S7: Mass spectrometric analysis: Comparison of MLGM vs. human adipocytes.
(a) SDS-PAGE separated and Coomassie blue-stained gels were used for mass spectrometric (MS) analysis. Lane areas at molecular weight of approximately 150kD were cut and used for MS (encircled in red): (1) human MLGM material (lane b); (2) total cell lysate of untreated preadipocytes (PO) (lane c); (3) total cell lysate of AIM-stimulated adipocytes additionally treated with oleic acid (P+OA) (lane d). The molecular weights of the reference proteins are indicated at the left margin. (b) Protein list of major MS results obtained from MLGM. (c) PO results. (d) P+OA results. Additional information is given within the box “Notes” (bottom left). Whereas MLGM revealed XOR, both adipocyte samples included AOX but not with XOR. These data confirmed the immunoblotting results (cp. Figs. 7, S6, for further aspects see also Discussion) (PNG 1.10 mb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heid, H., Zimbelmann, R., Dörflinger, Y. et al. Formation and degradation of lipid droplets in human adipocytes and the expression of aldehyde oxidase (AOX). Cell Tissue Res 379, 45–62 (2020). https://doi.org/10.1007/s00441-019-03152-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03152-1