Abstract
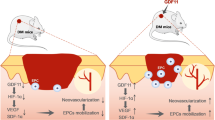
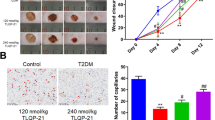
Diabetes mellitus (DM) often causes delayed wound healing in patients, which can lead to limb loss, disability, and even death. Many conventional therapeutic strategies have been proposed, but there is still no effective therapy for DM wounds. This study aimed to explore the effects of CD271 and phosphorylated tyrosine kinase receptor A (pTrkA) on the migration and proliferation abilities of epidermal stem cells (eSCs) and on the activation of DM wound healing. We investigated the interventional effects of CD271-overexpressing eSC (CD271 eSC) treatment and pTrkA inhibition (through k252a treatment) on delayed wound healing using mice with streptozotocin-induced DM. The migration and proliferation abilities of control eSCs, CD271 eSCs, and k252a-treated CD271 eSCs were observed under high-glucose conditions. Decreases in CD271 and increases in pTrkA were observed in DM mouse skin compared with control mouse skin; in addition, the rate of wound closure in DM mice was promoted by CD271 eSC treatment but delayed by pTrkA inhibition. Furthermore, the CD271 eSC migration and proliferation were greater than of control eSCs. Compared with that of CD271 eSCs, the number of CD271+k252a eSCs decreased significantly under high-glucose conditions. In parallel, the expression levels of the pERK, pAkt, and pJNK pathways increased in CD271 eSCs and decreased in CD271+k252a eSCs under high glucose. Our findings demonstrate that CD271 and pTrkA affect DM wound closure by promoting the eSC migration and proliferation. This mechanism involving the pERK, pAkt, and pJNK pathways might be a new therapeutic target for the treatment of delayed wound re-epithelialization in DM.








Similar content being viewed by others
Abbreviations
- DM:
-
Diabetes mellitus
- Egfr:
-
Epidermal growth receptor
- eSCs:
-
Epidermal stem cells
- GAPDH:
-
Glyceraldehydes-3-phosphate dehydrogenase
- High-Glu:
-
High glucose
- PI:
-
Propidium iodide
- STZ:
-
Streptozotocin
References
Al-Gayyar MM, Matragoon S, Pillai BA, Ali TK, Abdelsaid MA, El-Remessy AB (2011) Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes [corrected]. Diabetologia 54:669–680
Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascre G, Simons BD, Blanpain C (2017) Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun 8:14684
Baltzis D, Eleftheriadou I, Veves A (2014) Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther 31:817–836
Barker PA (2004) p75NTR is positively promiscuous: novel partners and new insights. Neuron 42:529–533
Blanpain C, Horsley V, Fuchs E (2007) Epithelial stem cells: turning over new leaves. Cell 128:445–458
Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD (1991) ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65:663–675
Cheng B, Liu HW, Fu XB, Sun TZ, Sheng ZY (2007) Recombinant human platelet-derived growth factor enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats. J Dermatol Sci 45:193–201
Cotsarelis G, Kaur P, Dhouailly D, Hengge U, Bickenbach J (1999) Epithelial stem cells in the skin: definition, markers, localization and functions. Exp Dermatol 8:80–88
Coulombe PA (2003) Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol 121:219–230
Delcroix JD, Michael GJ, Priestley JV, Tomlinson DR, Fernyhough P (1998) Effect of nerve growth factor treatment on p75NTR gene expression in lumbar dorsal root ganglia of streptozocin-induced diabetic rats. Diabetes 47:1779–1785
Driskell I, Oeztuerk-Winder F, Humphreys P, Frye M (2015) Genetically induced cell death in bulge stem cells reveals their redundancy for hair and epidermal regeneration. Stem Cells 33:988–998
Edwards JL, Vincent AM, Cheng HT, Feldman EL (2008) Diabetic neuropathy: mechanisms to management. Pharmacol Ther 120:1–34
Eming SA, Krieg T, Davidson JM (2007) Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 127:514–525
Fernyhough P, Diemel LT, Brewster WJ, Tomlinson DR (1994) Deficits in sciatic nerve neuropeptide content coincide with a reduction in target tissue nerve growth factor messenger RNA in streptozotocin-diabetic rats: effects of insulin treatment. Neuroscience 62:337–344
Frade JM, Rodriguez-Tebar A, Barde YA (1996) Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature 383:166–168
Fujimura M, Usuki F (2015) Methylmercury causes neuronal cell death through the suppression of the TrkA pathway: in vitro and in vivo effects of TrkA pathway activators. Toxicol Appl Pharmacol 282:259–266
Gambardella L, Barrandon Y (2003) The multifaceted adult epidermal stem cell. Curr Opin Cell Biol 15:771–777
Goren I, Muller E, Schiefelbein D, Gutwein P, Seitz O, Pfeilschifter J, Frank S (2009) Akt1 controls insulin-driven VEGF biosynthesis from keratinocytes: implications for normal and diabetes-impaired skin repair in mice. J Invest Dermatol 129:752–764
Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, Manni L, Madeddu P (2004) Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia 47:1047–1054
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Humpert PM, Kopf S, Djuric Z, Laine K, Korosoglou G, Rudofsky G Jr, Hamann A, Morcos M, von Eynatten M, Nawroth PP, Bierhaus A (2007) Levels of three distinct p75 neurotrophin receptor forms found in human plasma are altered in type 2 diabetic patients. Diabetologia 50:1517–1522
Kanazawa S, Fujiwara T, Matsuzaki S, Shingaki K, Taniguchi M, Miyata S, Tohyama M, Sakai Y, Yano K, Hosokawa K, Kubo T (2010) bFGF regulates PI3-kinase-Rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS One 5:e12228
Kaplan DR, Stephens RM (1994) Neurotrophin signal transduction by the Trk receptor. J Neurobiol 25:1404–1417
Kim MS, Kim YK, Eun HC, Cho KH, Chung JH (2006) All-trans retinoic acid antagonizes UV-induced VEGF production and angiogenesis via the inhibition of ERK activation in human skin keratinocytes. J Invest Dermatol 126:2697–2706
Lamers ML, Almeida ME, Vicente-Manzanares M, Horwitz AF, Santos MF (2011) High glucose-mediated oxidative stress impairs cell migration. PLoS One 6:e22865
Laverdet B, Danigo A, Girard D, Magy L, Demiot C, Desmouliere A (2015) Skin innervation: important roles during normal and pathological cutaneous repair. Histol Histopathol 30:875–892
Lim YC, Bhatt MP, Kwon MH, Park D, Na S, Kim YM, Ha KS (2015) Proinsulin C-peptide prevents impaired wound healing by activating angiogenesis in diabetes. J Invest Dermatol 135:269–278
Lima MH, Caricilli AM, de Abreu LL, Araujo EP, Pelegrinelli FF, Thirone AC, Tsukumo DM, Pessoa AF, dos Santos MF, de Moraes MA, Carvalheira JB, Velloso LA, Saad MJ (2012) Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS One 7:e36974
Mondal N, Pantalos G, Chen Z, Ding J, Sobieski M, Koenig S, Slaughter M, Wu Z (2016) Platelet activation, receptor shedding and aggregation in pediatric patients with congenital heart disease undergoing cardiopulmonary bypass. J Heart Lung Transplant 35:S399
Mysona BA, Al-Gayyar MM, Matragoon S, Abdelsaid MA, El-Azab MF, Saragovi HU, El-Remessy AB (2013) Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood-retina barrier breakdown in mice and rats. Diabetologia 56:2329–2339
Nykjaer A, Willnow TE, Petersen CM (2005) p75NTR--live or let die. Curr Opin Neurobiol 15:49–57
Organization WH (2015) The top 10 causes of death. Fact sheet 310 [updated May 2014].
Organization WH (2016) Global report on diabetes. World Health Organization
Sato H, Ebisawa K, Takanari K, Yagi S, Toriyama K, Yamawaki-Ogata A, Kamei Y (2015) Skin-derived precursor cells promote wound healing in diabetic mice. Ann Plast Surg 74:114–120
Sherman RA (2003) Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care 26:446–451
Shibata S, Tada Y, Asano Y, Hau CS, Kato T, Saeki H, Yamauchi T, Kubota N, Kadowaki T, Sato S (2012) Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J Immunol 189:3231–3241
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746
Somanath PR, Chen J, Byzova TV (2008) Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis 11:277–288
Tapley P, Lamballe F, Barbacid M (1992) K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 7:371–381
Terenghi G, Mann D, Kopelman PG, Anand P (1997) trkA and trkC expression is increased in human diabetic skin. Neurosci Lett 228:33–36
Torres OV, Jayanthi S, McCoy MT, Cadet JL (2018) Selective activation of striatal NGF-TrkA/p75NTR/MAPK intracellular signaling in rats that show suppression of methamphetamine intake 30 days following drug abstinence. Int J Neuropsychopharmacol 21:281–290
Trinh NT, Yamashita T, Ohneda K, Kimura K, Salazar GT, Sato F, Ohneda O (2016) Increased expression of EGR-1 in diabetic human adipose tissue-derived mesenchymal stem cells reduces their wound healing capacity. Stem Cells Dev 25:760–773
Vo PA, Tomlinson DR (1999) The regeneration of peripheral noradrenergic nerves after chemical sympathectomy in diabetic rats: effects of nerve growth factor. Exp Neurol 157:127–134
Wu KK, Huan Y (2008) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol Chapter 5:Unit 5.47
Xuan YH, Huang BB, Tian HS, Chi LS, Duan YM, Wang X, Zhu ZX, Cai WH, Zhu YT, Wei TM, Ye HB, Cong WT, Jin LT (2014) High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS One 9:e108182
Yu P, Wang Z, Sun X, Chen X, Zeng S, Chen L, Li S (2011) Hydrogen-rich medium protects human skin fibroblasts from high glucose or mannitol induced oxidative damage. Biochem Biophys Res Commun 409:350–355
Zhang M, Cao Y, Li X, Hu L, Taieb SK, Zhu X, Zhang J, Feng Y, Zhao R, Wang M, Xue W, Yang Z, Wang Y (2018a) Cd271 mediates proliferation and differentiation of epidermal stem cells to support cutaneous burn wound healing. Cell Tissue Res 371:273–282
Zhang M, Zhang Y, Ding J, Li X, Zang C, Yin S, Ma J, Wang Y (2018b) The role of TrkA in the promoting wounding-healing effect of CD271 on epidermal stem cells. Arch Dermatol Res 310:737–750
Zhu J, Wang P, Yu Z, Lai W, Cao Y, Huang P, Xu Q, Yu M, Xu J, Huang Z, Zeng B (2016) Advanced glycosylation end product promotes forkhead box O1 and inhibits Wnt pathway to suppress capacities of epidermal stem cells. Am J Transl Res 8:5569–5579
Ziegler D, Siekierka-Kleiser E, Meyer B, Schweers M (2005) Validation of a novel screening device (NeuroQuick) for quantitative assessment of small nerve fiber dysfunction as an early feature of diabetic polyneuropathy. Diabetes Care 28:1169–1174
Funding
Science and Technology Development Program of Shandong Province (No. 2016GSF201080), and National Natural Science Foundation of China (No. 81772092 and 81571911) supported this study.
Author information
Authors and Affiliations
Contributions
Yibing Wang designed the experiments; Xiaohong Li, Min Zhang, and Mengyao Liu performed the experiments; Rui Zhang, Zhang Feng, Jiaxu Ma, Huayu Zhang, Kaifeng Huang, and Mengyao Liu contributed reagents, materials, analysis tools, added, and repeated the animal experiments; Mengyao Liu also edited the manuscript. Yongqian Cao and Siyuan Yin analyzed the data; Jun Ding, Xiaohong Li, Mengyao Liu, and Min Zhang wrote and reviewed the paper.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there are no conflicts of interests.
According to the National Institutes of Health (NIH) Guide, all surgeries and experiments were performed under the pentobarbital sodium anesthesia, and followed the rules of the Committee on the Ethics of Shandong University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary figure 1:
HE staining and immunohistochemical staining in normal control mouse skin and DM-control mouse skin. (a, b) HE staining showed that the epidermal layer was thinner in DM-control mice than in normal-control mice.Bar=120 μm. (a’, b’) Immunohistochemical staining revealed that CD271 was expressed at lower levels in DM-control mouse epidermal skin than in normal-control mouse epidermal skin. Bar=120 μm. (PNG 430 kb)
Supplementary figure 2:
Schematic of the effects of injected eSCs on the wound healing model. (PNG 2178 kb)
Supplementary figure 3:
Expression levels of CD271 in control eSCs, CD271 (CD271-overexpressing) eSCs, and CD271+k252a eSCs cultured with High-Glu-containing KSFM. a WB analysis of the protein expression of CD271. b The expression levels were calculated relative to those of the internal control GAPDH (ANOVA, *p<0.05, n=3). (PNG 418 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Zhang, R., Li, X. et al. CD271 promotes STZ-induced diabetic wound healing and regulates epidermal stem cell survival in the presence of the pTrkA receptor. Cell Tissue Res 379, 181–193 (2020). https://doi.org/10.1007/s00441-019-03125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03125-4