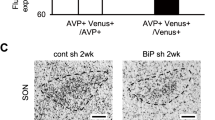
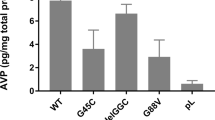
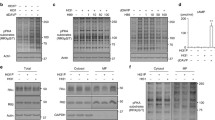
Abstract
Familial neurohypophysial diabetes insipidus (FNDI), characterized by delayed-onset progressive polyuria and loss of arginine vasopressin (AVP) neuron, is an autosomal dominant disorder caused by AVP gene mutations. We previously generated a knock-in mouse model for FNDI, which recapitulated the phenotype of human FNDI. To address the mechanisms underlying AVP neuron loss, we subjected FNDI mice to intermittent water deprivation, which accelerated the phenotype and induced AVP neuron loss within a relative short period. Electron microscopic analyses revealed that aggregates were confined to a sub-compartment of the endoplasmic reticulum (ER), ER-associated compartment (ERAC), in AVP neurons of FNDI mice under normal conditions. In contrast, aggregates scattered throughout the dilated ER lumen, and phagophores, autophagosome precursors, emerged and surrounded the ER containing scattered aggregates in FNDI mice subjected to water deprivation for 4 weeks, suggesting that failure of ERAC formation leads to autophagy induction for degradation of aggregates. Furthermore, the cytoplasm was entirely occupied with large vacuoles in AVP neurons of FNDI mice subjected to water deprivation for 12 weeks, at which stage 30–40% of AVP neurons were lost. Our data demonstrated that although autophagy should primarily be a protective mechanism, continuous autophagy leads to gradual loss of organelles including ER, resulting in autophagy-associated cell death of AVP neurons in FNDI mice.



Similar content being viewed by others
References
Arima H, Kondo K, Kakiya S, Nagasaki H, Yokoi H, Yambe Y, Murase T, Iwasaki Y, Oiso Y (1999) Rapid and sensitive vasopressin heteronuclear RNA responses to changes in plasma osmolality. J Neuroendocrinol 11:337–341
Arima H, Azuma Y, Morishita Y, Hagiwara D (2016) Central diabetes insipidus. Nagoya J Med Sci 78:349–358
Babey M, Kopp P, Robertson GL (2011) Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol 7:701–714
Bergeron C, Kovacs K, Ezrin C, Mizzen C (1991) Hereditary diabetes insipidus: an immunohistochemical study of the hypothalamus and pituitary gland. Acta Neuropathol 81:345–348
Bernal A, Mahía J, Puerto A (2016) Animal models of central diabetes insipidus: human relevance of acquired beyond hereditary syndromes and the role of oxytocin. Neurosci Biobehav Rev 66:1–14
Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4:e423
Beuret N, Hasler F, Prescianotto-Baschong C, Birk J, Rutishauser J, Spiess M (2017) Amyloid-like aggregation of provasopressin in diabetes insipidus and secretory granule sorting. BMC Biol 15:5
Birk J, Friberg MA, Prescianotto-Baschong C, Spiess M, Rutishauser J (2009) Dominant pro-vasopressin mutants that cause diabetes insipidus form disulfide-linked fibrillar aggregates in the endoplasmic reticulum. J Cell Sci 122:3994–4002
Bisset GW, Chowdrey HS (1988) Control of release of vasopressin by neuroendocrine reflexes. Q J Exp Physiol 73:811–872
Braverman LE, Mancini JP, McGoldrick DM (1965) Hereditary idiopathic diabetes insipidus. A case report with autopsy findings. Ann Intern Med 63:503–508
Brownstein MJ, Russell JT, Gainer H (1980) Synthesis, transport, and release of posterior pituitary hormones. Science 207:373–378
Bruins J, Kovács GL, Abbes AP, Burbach JP, van den Akker EL, Engel H, Franken AA, de Wied D (2006) Minor disturbances in central nervous system function in familial neurohypophysial diabetes insipidus. Psychoneuroendocrinology 31:80–91
Burbach JP, Luckman SM, Murphy D, Gainer H (2001) Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev 81:1197–1267
Castino R, Davies J, Beaucourt S, Isidoro C, Murphy D (2005a) Autophagy is a prosurvival mechanism in cells expressing an autosomal dominant familial neurohypophyseal diabetes insipidus mutant vasopressin transgene. FASEB J 19:1021–1023
Castino R, Isidoro C, Murphy D (2005b) Autophagy-dependent cell survival and cell death in an autosomal dominant familial neurohypophyseal diabetes insipidus in vitro model. FASEB J 19:1024–1026
Castino R, Thepparit C, Bellio N, Murphy D, Isidoro C (2008) Akt induces apoptosis in neuroblastoma cells expressing a C98X vasopressin mutant following autophagy suppression. J Neuroendocrinol 20:1165–1175
Chini B, Verhage M, Grinevich V (2017) The action radius of oxytocin release in the mammalian CNS: from single vesicles to behavior. Trends Pharmacol Sci 38:982–991
Daughters K, Manstead ASR, Rees DA (2017) Hypopituitarism is associated with lower oxytocin concentrations and reduced empathic ability. Endocrine 57:166–174
Davies J, Murphy D (2002) Autophagy in hypothalamic neurones of rats expressing a familial neurohypophysial diabetes insipidus transgene. J Neuroendocrinol 14:629–637
Granell S, Baldini G, Mohammad S, Nicolin V, Narducci P, Storrie B (2008) Sequestration of mutated alpha1-antitrypsin into inclusion bodies is a cell-protective mechanism to maintain endoplasmic reticulum function. Mol Biol Cell 19:572–586
Green JR, Buchan GC, Alvord EC, Swanson AG (1967) Hereditary and idiopathic types of diabetes insipidus. Brain 90:707–714
Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B (2016) Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol Psychiatry 79:155–164
Hagiwara D, Arima H, Morishita Y, Wenjun L, Azuma Y, Ito Y, Suga H, Goto M, Banno R, Sugimura Y, Shiota A, Asai N, Takahashi M, Oiso Y (2014) Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus. Cell Death Dis 5:e1148
Hayashi M, Arima H, Ozaki N, Morishita Y, Hiroi M, Nagasaki H, Kinoshita N, Ueda M, Shiota A, Oiso Y (2009) Progressive polyuria without vasopressin neuron loss in a mouse model for familial neurohypophysial diabetes insipidus. Am J Physiol Regul Integr Comp Physiol 296:R1641–R1649
Hiroi M, Morishita Y, Hayashi M, Ozaki N, Sugimura Y, Nagasaki H, Shiota A, Oiso Y, Arima H (2010) Activation of vasopressin neurons leads to phenotype progression in a mouse model for familial neurohypophysial diabetes insipidus. Am J Physiol Regul Integr Comp Physiol 298:R486–R493
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. N Engl J Med 361:1570–1583
Huyer G, Longsworth GL, Mason DL, Mallampalli MP, McCaffery JM, Wright RL, Michaelis S (2004) A striking quality control subcompartment in Saccharomyces cerevisiae: the endoplasmic reticulum-associated compartment. Mol Biol Cell 15:908–921
Ito M, Jameson JL (1997) Molecular basis of autosomal dominant neurohypophyseal diabetes insipidus. Cellular toxicity caused by the accumulation of mutant vasopressin precursors within the endoplasmic reticulum. J Clin Invest 99:1897–1905
Ito M, Yu RN, Jameson JL (1999) Mutant vasopressin precursors that cause autosomal dominant neurohypophyseal diabetes insipidus retain dimerization and impair the secretion of wild-type proteins. J Biol Chem 274:9029–9037
Ito D, Yagi T, Ikawa M, Suzuki N (2012) Characterization of inclusion bodies with cytoprotective properties formed by seipinopathy-linked mutant seipin. Hum Mol Genet 21:635–646
Kamimoto T, Shoji S, Hidvegi T, Mizushima N, Umebayashi K, Perlmutter DH, Yoshimori T (2006) Intracellular inclusions containing mutant alpha1-antitrypsin Z are propagated in the absence of autophagic activity. J Biol Chem 281:4467–4476
Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V (2012) Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73:553–566
Kroemer G, Levine B (2008) Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9:1004–1010
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G, NCoCD (2009) Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ 16:3–11
Nagai I, Li CH, Hsieh SM, Kizaki T, Urano Y (1984) Two cases of hereditary diabetes insipidus, with an autopsy finding in one. Acta Endocrinol 105:318–323
Nagasaki H, Ito M, Yuasa H, Saito H, Fukase M, Hamada K, Ishikawa E, Katakami H, Oiso Y (1995) Two novel mutations in the coding region for neurophysin-II associated with familial central diabetes insipidus. J Clin Endocrinol Metab 80:1352–1356
Nijenhuis M, Zalm R, Burbach JP (1999) Mutations in the vasopressin prohormone involved in diabetes insipidus impair endoplasmic reticulum export but not sorting. J Biol Chem 274:21200–21208
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Pierzynowska K, Gaffke L, Cyske Z, Puchalski M, Rintz E, Bartkowski M, Osiadły M, Pierzynowski M, Mantej J, Piotrowska E, Węgrzyn G (2018) Autophagy stimulation as a promising approach in treatment of neurodegenerative diseases. Metab Brain Dis. https://doi.org/10.1007/s11011-018-0214-6
Russell TA, Ito M, Yu RN, Martinson FA, Weiss J, Jameson JL (2003) A murine model of autosomal dominant neurohypophyseal diabetes insipidus reveals progressive loss of vasopressin-producing neurons. J Clin Invest 112:1697–1706
Si-Hoe SL, De Bree FM, Nijenhuis M, Davies JE, Howell LM, Tinley H, Waller SJ, Zeng Q, Zalm R, Sonnemans M, Van Leeuwen FW, Burbach JP, Murphy D (2000) Endoplasmic reticulum derangement in hypothalamic neurons of rats expressing a familial neurohypophyseal diabetes insipidus mutant vasopressin transgene. FASEB J 14:1680–1684
Swanson LW, Sawchenko PE (1983) Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6:269–324
Teckman JH, Perlmutter DH (2000) Retention of mutant alpha(1)-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol 279:G961–G974
Valetti C, Grossi CE, Milstein C, Sitia R (1991) Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J Cell Biol 115:983–994
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281:30299–30304
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hagiwara, D., Grinevich, V. & Arima, H. A novel mechanism of autophagy-associated cell death of vasopressin neurons in familial neurohypophysial diabetes insipidus. Cell Tissue Res 375, 259–266 (2019). https://doi.org/10.1007/s00441-018-2872-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2872-4