Abstract
Proteomic and genomic techniques have reached full maturity and are providing unforeseen details for the comprehensive understanding of disease pathologies at a fraction of previous costs. However, for kidney diseases, many gaps in such information remain to inhibit major advances in the prevention, treatment and diagnostics of these devastating diseases, which have enormous global impact. The discovery of ubiquitous extracellular vesicles (EV) in all bodily fluids is rapidly increasing the fundamental knowledge of disease mechanisms and the ways in which cells communicate with distant locations in processes of cancer spread, immunological regulation, barrier functions and general modulation of cellular activity. In this review, we describe some of the most prominent research streams and findings utilizing urinary extracellular vesicles as highly versatile and dynamic tools with their extraordinary protein and small regulatory RNA species. While being a highly promising approach, the relatively young field of EV research suffers from a lack of adherence to strict standardization and carefully scrutinized methods for obtaining fully reproducible results. With the appropriate guidelines and standardization achieved, urine is foreseen as forming a unique, robust and easy route for determining accurate and personalized disease signatures and as providing highly useful early biomarkers of the disease pathology of the kidney and beyond.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following extensive genome-wide analyses and the full maturation of integrative technologies such as RNA-sequencing (RNA-Seq) techniques to reveal the crucial regulatory functions of the “non-coding” DNA bulk of human and other species (Lee et al. 2010), recent years have seen strong advances in a variety of organ functions with unprecedented molecular accuracy. Nevertheless, an extensive understanding of, for example, key kidney functions, including mechanisms of glomerular filtration and the formation of urine from primary urine to the final voided urine, remains to be achieved (Saritas et al. 2015; Maas et al. 2016). The combined use of proteomics, lipidomics, metabolomics and comprehensive genomic analyses, together with advanced systems biology algorithms, offers the promise of major new discoveries for the benefit of patients with kidney disease.
Urine formation provides a key route for waste removal from the body. In addition to immediate metabolic waste products, variable amount of electrolytes, small peptides and larger functional proteins (Hildonen et al. 2016) are removed. Based, for example, on hydration status, diet and excercise, the electrolyte concentration may variate considerably. For years, this dynamic nature of urine has presented a challenge for the full exploitation of the potential of this bodily fluid, which is easy to collect.
The structural basis of glomerular filtration has been defined down to the fine molecular level, including the identification of the respective signaling and functional pathways, whereas the functional mechanisms remain to be fully understood (Patrakka and Tryggvason 2010; Pollak et al. 2014; Scott and Quaggin 2015). At the same time, however, the continuous global increase of chronic kidney diseases (CKD) attributable to a variety of causes involves over 10 % of most populations and geographical areas (El Nahas 2005; Remuzzi et al. 2006). These alarming numbers call for practical advances leading to early diagnostics, better therapies and cost-efficient personalized disease management even in less fortunate areas and countries. Many of the technologies required are beginning to reach their full maturity for the achievement of these goals.
The increase of CKD is mostly the result of the rapid increase of diabetes world-wide (El Nahas 2005). However, patient subpopulations susceptible to life-threatening diabetic end-organ damage mostly cannot be identified early enough to prevent the progress of disease. Apart from the medicinal modulation of the renin-angiotensin axis (Remuzzi et al. 2006), only a few other CKD disease mechanisms have been identified and, accordingly, the repertoire of targeted medication available to halt disease progression is not satisfactory. Currently, the exhaustive genetic data produced have provided few clues to the factors and mechanisms for the identification of the vulnerable target subpopulations or even of those who might benefit from the currently available treatment options. In spite of numerous proposed early biomarkers to monitor disease progression and activity, none have been fully validated for wide clinical use.
The discovery of vesicles of various sizes secreted from practically all cell types in the body (Thery et al. 2009; Yanez-Mo et al. 2015) is rapidly revolutionizing our understanding of key biological phenomena including organ growth and development, cancer and metastasis, cellular homeostasis, immunological defense, barrier functions to the exterior and, importantly, intercellular communication (for references, see Thery et al. 2009, van der Pol et al. 2012). Although this is a relatively young field, many new cellular-intercellular mechanisms including the spread of viral infections (Nour and Modis 2014) and the spread of cancer cells (Tomasetti et al. 2017) can be explained by messages from extracellular vesicles (EV).
Extracellular vesicles
EV are a heterogenous group of membrane-coated particles of various sizes (Fig.1) either actively or passively secreted from cells by well-established mechanisms (Thery et al. 2009; van der Pol et al. 2012; Akers et al. 2013; Ciardiello et al. 2016; Morrison et al. 2016; Table 1).
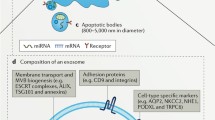
Biogenesis of microRNA (miRNA), extracellular vesicles (EV) and uptake mechanisms. miRNAs are transcribed by RNA polymerase II from chromosomal DNA into primary RNA (Pri-miRNA; 1-3 kb). Pri-miRNA is processed by Drosha into precursor miRNA (Pre-miRNA). Pre-miRNA is transported to the cytoplasm and cleeved into miRNA/miRNA duplices (∼22 bp) by Dicer. miRNA duplex strands separate with the incorporation of the protein Argonaute (AGO) and RNA-induced silencing complex (Risk; Xu et al. 2013; Sohel 2016; Tomasetti et al. 2017). miRNA can be packed in the EV or be exported as protein-miRNA complexes (Arroyo et al. 2011; Canfran-Duque et al. 2014). Exosomes are derived from the endocytic pathway and their biogenesis requires multiprotein complexes called Endosomal Sorting Complex Required for Transport. Microvesicles are formed by the outward budding of the plasma membrane, whereas apoptotic bodies are vesicles released from cells that undergo apoptosis (Akers et al. 2013; Morrison et al. 2016). EV are taken up by recipient cells by a variety of mechanisms including endocytosis (mediated by lipid rafts, clathrin or caveolin), pinocytosis, phagocytosis and membrane fusion (Mulcahy et al. 2014). Proteins present on the EV can trigger signaling pathways in the target cells or be involved in antigen presentation (Thery et al. 2009; El Andaloussi et al. 2013). miRNA-protein complexes are also internalized by interaction with specific receptors on the recipient cell. High-density lipoprotein (HDL) associated with miRNA (miRNA-HDL) interacts with scavenger receptor class B type 1 (SR-B1; Canfran-Duque et al. 2014). miRNA-AGO-2 interacts with neuropilin-1 (NRP1; Prud’homme et al. 2016). EE Early endosome, MVB multivesicular body, NPM1 nucleophosmin 1, OMVs outer membrane vesicles, dsRNA double-stranded RNA
EVs have a distinct surface coat with an abundance of membrane-associated proteins, glycoproteins and lipids, whereas the EV interior (“cargo”) consists in structural and functional proteins, enzymes, lipids and peptides of various lengths. Notably, DNA (including mitochondrial DNA) and a variety of RNA species are transported within EVs. These important RNA classes have now been convincingly shown to regulate all cellular functions efficiently and include small RNAs, microRNAs and messager RNAs (Thery et al. 2009; El Andaloussi et al. 2013; Liu et al. 2015; Zaborowski et al. 2015; Morrison et al. 2016), see Fig. 1. Originally mostly ignored in electron micrographs or described as “cell-derived dust” from megakaryocytes (Wolf 1967), their original role was supposed to be passive cellular waste management. However, intriguing findings of active and orderly secretion mechanisms have emerged during the last few years. Interestingly, a wealth of information is now available that confirms that the vesicles are indeed taken up by target cells via a variety of mechanisms (summarized in Mulcahy et al. 2014) revealing their key role in intercellular communication, although targeted uptake mechanisms in any particular organ systems, except for the brain, remain to be studied in detail.
Crude urine or urinary EV (uEV), with their protein, enzyme or RNA content, have been discovered over the last few years (Alter et al. 2012; Alvarez et al. 2012; Cheng et al. 2014). In this review, we summarize some recent discoveries and highlight especially the distinct value of urinary RNA for biomarker purposes (Alter et al. 2012; Alvarez and Distefano 2013; Yang et al. 2013; Argyropoulos et al. 2015). With all the present data available, especially of the surprisingly rich and variable content of uEV, urine now appears as a very promising and completely uninvasive source for new information reflecting accurately the pathophysiology of the kidney and, most likely, also of other organ systems. Despite all the enthusiasm in this rapidly growing field, we aim to pinpoint some of the caveats, misinterpretations and limitations of current approaches and to emphasize the importance of the appropriate standardization needed.
EV classification
Since the first descriptions of EV, a variety of vesicle categories has been described. The classification was based initially on their cellular or subcellular sources, such as prostasomes, exosomes, membrane vesicles and others (see van der Pol et al. 2012, 2016; Thery et al. 2009; Wang and Sun 2014) and basically their physico-chemical properties. These include the vesicle size, density, morphology, lipid composition, protein composition, subcellular origin and light scattering (Thery et al. 2009; van der Pol et al. 2012). As seen in Table 1, most of the physico-chemical EV characteristics overlap significantly. This also makes a reliable categorization and their isolation a challenge yet to be fully overcome. Notably, in addition to the size and density of vesicles, the efficiency of isolating these vesicles depends on the shape and volume fraction of the vesicles, the viscosity of the fluid in which they lie, the temperature and presence of other confounding factors such as proteins, peptides or pigments in the fluid, the centrifugation time and the type of rotor used for centrifugation (fixed angle or swing-out; Cvjetkovic et al. 2014; Livshits et al. 2015). Based on these variables and the current lack of thoroughly standardized isolation protocols, considerable cross-contamination of vesicle types can obviously occur in any sample studied and reported. Indeed, this overlap between vesicle categories and the variety of isolation methods might have led to “published artifacts, over-interpretation and non-comparable results between laboratories” (van der Pol et al. 2016), despite continuous attempts at standardization by international organizations (Witwer et al. 2013). On the other hand, especially for biomarker identification, the current trend is leaning towards the use of methods providing the best total EV yield, which may ignore strict EV categorization. Depending on downstream uses and goals, this should be considered acceptable, in particular as most vesicle classes share, to a significant degree, the same surface and cargo contents (even if in different ratios).
As stated above, urine contains a variety of vesicles of variable sizes (Pisitkun et al. 2004; Miranda et al. 2010; van Balkom et al. 2011; Alvarez et al. 2012; Alvarez et al. 2013). Notably, however, urinary contents, including vesicles can be strongly influenced by factors such as diet (Garcia-Perez et al. 2017), exercise (Alter et al. 2012; Yang et al. 2013; Mansueto et al. 2017) and medication and by the presence of urinary pigments. As discussed below in more detail, the lack of standardization and variables in nomenclature might have led to reported artifacts and challenges of reproducibility. Furthermore, free urine contains free and active proteases and RNases, whereas within the vesicles, both proteins and RNA are protected against ubiquitous proteases and RNases (Cheng et al. 2014). Thus, only results of crude urines are comparable with each other, whereas studies utilizing the much richer contents of EV should be preferred because of their mostly unmodified contents.
Interestingly, the bulk of urinary vesicles is considered to derive from the epithelial lining of nephrons and kidney parenchyme (Turco et al. 2016) but ample evidence has been presented that urinary vesicles also originate from the circulation (Miranda et al. 2010; Ma et al. 2016; Pazourkova et al. 2016). This offers an exciting opportunity to develop easy, non-invasive and easy-to-repeat diagnostics for remote tissue-specific markers (Ma et al. 2016) present in the urine. However, whether uEVs are actively secreted through the glomerular filtration barrier or through the epithelial cell lining of nephrons is not known, although this could involve many active mechanisms (for a variety of the mechanisms proposed, see Mulcahy et al. 2014).
Evidence is increasing for uEV usefulness in the biomarker search for kidney diseases such as minimal change disease and focal segmental glomerulosclerosis (Ramezani et al. 2015), diabetic nephropathy (Barutta et al. 2013; Musante et al. 2015; Delic et al. 2016) and others (for an excellent comprehensive review of uEV findings in kidney diseases, see Erdbrugger and Le 2016).
Although proteins within uEVs have been widely recognized and reported (see, for example, www.exocarta.org, the dedicated database for vesicle contents), the recent technical advances in RNA-sequencing (Wang et al. 2009; Lee et al. 2010) highlight the usefulness especially of urinary miRNA for biomarker purposes (Van Roosbroeck et al. 2013). Interestingly, both uEV proteins and miRNAs are products that not only are secreted by all the epithelial cell types along the nephrons and lower urinary tract but are also filtered or secreted from the circulation by the kidney parenchyme.
In contrast to earlier studies also utilizing urinary sediment cells for miRNA isolation, the protocols presently call for them being discarded as these most likely represent contents from apoptotic cells. The information available concerning specific downstream target effects of uEV miRNAs remains limited (however, see Bellingham et al. 2012; Alvarez-Erviti et al. 2011).
Interestingly, evidence is accumulating of yet another potential source of urinary RNA, namely the resident bacteria (Table 2). Accordingly, inherent but well-constrained bacterial colonization can be found in the urinary bladder (Brubaker and Wolfe 2015). For anatomical reasons, the female urinary bladder and urine are more prone to urinary tract infections (UTI). Whether this represents an escape of normal bacterial flora from the host or the entry of more pathogenic urinary pathogens, such as the uropathogenic fimbriated strains of Escherichia coli (Korhonen et al. 1988), remains unknown. Interestingly, the most common bacterial strains in urinary bladder associated with UTI (both Gram negative and Gram positives) have been well-established, whereas recent studies by using RNA-Seq of the 16S RNA isolated from urine have shown a wide variety of additional bacterial strains in urine (Valadi et al. 2007; Brubaker and Wolfe 2015; Koeppen et al. 2016; Tataruch-Weinert et al. 2016). At the same time, bacteria have been shown to actively use their outer membrane vesicles (OMVs) for packaging and sending their genetic material for interaction with the immediate environment. The OMV contents also include abundant small RNA species (Wang et al. 2012; Koeppen et al. 2016), which mediate interactions with host cells and tissues. Thus, urine unavoidably also contains vesicles from rich bacterial sources, with their respective RNA contents. Although little is still known of the exact roles of OMVs, they are clearly involved in the constant interplay with the host defense system and in maintaining the barrier function to prevent ascending infections.
For practical purposes in uEV studies, the OMVs and their protein and RNA content are of special importance. Several questions arise. How can excessive bacterial products in the analyses and respective artifacts in the reported proteomes and RNA be avoided (Shmaryahu et al. 2014; Cheung et al. 2016)? Should samples from male and female subjects be differently interpreted as male samples present with less abundant bacteria and their OMV products? Carefulness at all steps in sample collection, storage, analysis and data interpretation is mandatory and, notably, calls for rigorous technical controls to pinpoint excessive bacterial RNA and protein products. This, in turn, may require a much larger volume of urine samples to allow all the necessary orthogonal controls (Tataruch-Weinert et al. 2016).
The capacity of bacteria to exchange functional genetic information between host cells is a widely unexplored area that has profound repercussions in our understanding of clinical UTI and beyond. The presence of bacterial proteins, metabolites and RNA products may generate misleading data and this possibility should always be critically considered in studies with uEV analytics.
Isolation methods of uEV have been extensively reviewed (Musante et al. 2014b; Wang and Sun 2014; Gamez-Valero et al. 2015). The most widely used methods are still the differential (ultra)centrifugation–based methods, often with modifications (see Table 3; Gardiner et al. 2016).
To isolate urinary EVs, a minimum of two steps are usually used, including low-speed centrifugation to pellet any cellular debris in the urine for discard.
Variations and combinations of other methods as “add-ons” to differential centrifugation have been introduced to overcome the observed limitations, including the aggregation and unnecessary loss of valuable pellets still containing EVs during the process. Notably, a number of practical parameters for the ultracentrifugation-based methods should always be carefully considered and details including relative centrifugal force, centrifugation time, temperature and the rotor type used should be recorded since variation in these parameters will lead to substantial quantitative and qualitative differences in the final EV yield (Thery et al. 2009). A detailed discussion of caveats in many of the currently used protocols can be found in recent critical reviews (Gardiner et al. 2016; van der Pol et al. 2016).
Reducing agents including dithiothreitol (DTT) or CHAPS (3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate) are used to prevent excessive protein complexing during EV isolation, especially in order to regulate polymerization of Tamm-Horsfall glycoprotein (THP; Fernandez-Llama et al. 2010; Musante et al. 2012), the most abundant normal urinary protein distorting many downstream analyses. Notably, an intact urinary THP meshwork efficiently entraps vesicles of all sizes at all steps of EV harvesting, causes clogging and a seriously reduced isolation capacity of in-filtration-based methods and may result in a loss of up to 30 % of the final uEV yield (Fernandez-Llama et al. 2010). Notably, however, the use of reducing agents may also release miRNA bound to protective circulating proteins or from the surface of uEVs (Wachalska et al. 2016).
The current golden standard, namely serial (ultra)centrifugation, has been shown to easily miss up to 20–30 % of vesicles in the pre-purification steps (Musante et al. 2014a). Furthermore, recent results demonstrate that the final pellet after the ultracentrifugation steps for vesicle isolation may have missed yet another 20–30 % of vesicles (Musante et al. 2017). These alarming facts should be carefully taken into consideration in order to include EV isolation methods that have been selected and modified and published results that have been critically evaluated.
The most commonly used current methods and their combinations for EV isolation from urine are listed in Table 3. Notably, many of these methods can easily clog because of protein aggregates or the capacity of the method might be limited. For practical isolation purposes and for the amounts necessary especially for uEVs, large sample volumes are a definite benefit as this allows the inclusion of the necessary quality control of the samples. An additional source of artifacts, namely variations in urinary electrolyte concentrations, e.g., resulting from hydration status because of exercise (Maughan 1991), may likewise change the sample conditions and distort EV yield. Furthermore, urine is abundant in pigments, a recognized major source of artifacts in all downstream analyses. An interesting approach might be to use fluorescence-activated cell sorting (FACS) for direct vesicle isolation into various categories, although the resolution of FACS is presently limited mostly to vesicle size above 100 nm and thus misses a major part of of vesicles, especially exosomes.
Musante et al. (2014a) developed an alternative isolation method for the comprehensive catching of urinary vesicles. This method is called hydrostatic filtration dialysis (HFD) and avoids the recognized limitations of most EV isolation methods. It uses simple and quick low-speed centrifugation (2000 g) to remove, for example, bacteria, cellular debris and excessive polymers of THP before filter-dialysing the sample (especially urine).
In HFD, the hydrostatic pressure of the sample pushes it through the dialysis membrane tubing. For recovery of urinary proteins and miRNA, the HFD method is clearly superior to the ultracentrifugation-based methods and provides superior quality of protein and RNA yield (Musante et al. 2014a; Tataruch-Weinert et al. 2016).
HFD is versatile, simple and inexpensive and can be used for a variety of samples including urine, plasma, cell culture medium and saliva (Musante et al. 2014a). It is based on sample dialysis in a tube with a defined-cutoff pore-size membrane (Musante et al. 2012). This membrane allows the passage of small solutes and peptides, effectively standardizing the solute environment, while retaining the EVs because of their larger size. HFD easily provides normalization and collection of the yield and washes away electrolytes while efficiently retaining the vesicles. Notably, the major contaminant in all urinary downstream analyses, namely urinary pigments, are also washed though the membrane and thus are avoided. The capacity of this method is adequate, with up to 1000 ml sample volumes being easily handled. Furthermore, this method requires little or no previous knowledge or training, is extremely cost-efficient and can readily process a number of samples in parallel during a workday (Musante et al. 2014a). The HFD method is not suitable for distinguishing between defined vesicle populations, which, for most diagnostic, prognostic or biomarker searches is, however, irrelevant.
Antibodies and synthetic peptides with affinity for EV membrane proteins have been used for EV isolation (Ghosh et al. 2014; Wang and Sun 2014). Although these methods may preferentially yield exosomes or other EV types with distinct surface antigens as required, additional EV subpopulations sharing the same membrane proteins may be isolated. Moreover, their capacity for EV catching is limited making them more suitable for smaller sample volumes.
Specific kits to precipitate exosomes have been developed, e.g., ExoSpin Exosome Purification Kit (Cell Guidance Systems, USA), Invitrogen Total Exosome Isolation Kit (Life Technologies, USA) and others. These products have been tested with solutions of liposomes and exosomes and have shown not only a robust isolation capacity for EV but also the co-isolation of molecules with similar physical properties. Thus, other additional purification methods may need to be used (Lane et al. 2015).
RNA species in vesicles
Intracellular RNA species are involved in the translation of DNA information to proteins at ribosomes and in the general regulation of RNA translation (see Grosshans and Filipowicz 2008, Xu et al. 2013). MiRNA, with the size of 20-25 nt, has a distinct role in gene regulation (Xu et al. 2013). Its formation is regulated by a specific intracellular pathway distinguishing it from other small RNA species present both in pro- and eukaryotic cells (Xu et al. 2013; see Fig. 1).
Interestingly, all RNA classes can also be found extracellularly, in all bodily fluids, including serum and plasma, saliva, tear fluid and urine (Wang et al. 2012; Patton et al. 2015; Yanez-Mo et al. 2015). The way in which the cellular RNA secretion itself is regulated and the relative proportions of the various small RNA species secreted are not exactly known. The intracellular RNA profile may differ from that of the secreted profile, suggesting the possibility of the overflow being secreted. Packaged into EV but also free, RNA appears as a unique and archaic method of cell-to-cell communication found throughout living cells (Brown et al. 2015; Yanez-Mo et al. 2015). Interestingly, the RNA secretome appears to reflect accurately the physiological state of the cell of origin and is highly valuable and accurate for the mapping of any personalized functions.
Although free extracellular RNA was identified years ago, the extracellular milieu consists in complex proteases and ubiquitous RNases actively splicing any target secreted from the cells (Sorrentino 1998; DeClerck et al. 2004; Ricard-Blum and Vallet 2016). The splicing process produces the extracellular fluid proteome (peptidome) and RNome. What is even more exciting is that cells ranging from archaic bacteria to the most highly sophisticated cell types have developed methods to protect these valuable entities from degradation by packaging them into vesicles and, indeed, extracellular RNAs appear to be universally associated instead with carrier vehicles (Patton et al. 2015), probably because of the rapid degradation of unprotected RNAs in biofluids.
Exosomes appear as a dominant pathway and vehicle for RNA secretion from cells (Crescitelli et al. 2013; Lunavat et al. 2015; Willms et al. 2016), although other vesicle types also contain RNA. This fact reduces the value of any method designed strictly for the purification of a distinct EV class. Moreover, these methods always end up in losing up in 40 % of EV because of losses in the process up until the last separation steps (Musante et al. 2017). Furthermore, the membrane layer of all vesicle types has been shown to contain a distinct surface coat that may be quite different from the vesicle interior, i.e., the “vesicle cargo” (Liu et al. 2015). This is interesting, as many lines of evidence now suggest different roles for these regions: the surface proteins and even EV surface RNA may serve as an “address code” for targeting (for references, see Mulcahy et al. 2014). As the vesicle attaches and fuses with the target cell membrane, its contents are released for intracellular interaction. This appears as a finely tuned and highly sophisticated system for target cell regulation in processes such as immunological defense, cancer spread and the modulation of the metabolic state of cells in a specific manner (El Andaloussi et al. 2013; Yanez-Mo et al. 2015; Zaborowski et al. 2015).
The gut, skin, urine and mucous membrane microbiomes appear to have developed methods to efficiently interfere with the host defense systems by releasing outer membrane vesicles (OMVs), with distinct RNA contents and constant interaction with the host defense system. As a result, a strong, although less-well understood barrier function is achieved.
Future aspects
The number of studies on EV, especially exosomes, has been sky-rocketing during the last few years. This is because their extraordinary potential is not limited to the dissection of disease mechanisms to identify new druggable targets but can also provide early biomarkers of unforeseen accuracy. EV, which are also abundantly present in the urine, are an unparalleled resource for individualized molecular fingerprints reflecting cellular pathophysiology at their site of origin upstream in the kidney and beyond (Miranda et al. 2010; Alvarez and Distefano 2013). Thus, EV are derived not only from all cell types along the nephron and kidney parenchyme (Salih et al. 2014) but also from the circulation and normal bacterial colonization in the urinary bladder.
The potential of EV, especially for early diagnostics and as novel biomarkers for kidney diseases, is exciting. With full systems biology integration, combining their proteome, RNome and metabolomics information, EV now show great promise in dissecting these thus far less-well understood disease mechanisms in an individualized way (Erdbrugger and Le 2016; van der Pol et al. 2016). The field still lacks comprehensive standardization, starting from the nomenclature and methods used for efficient EV harvesting and extending up to their critical downstream analysis. Misleading or false results in the field may lead to wrong conclusions (van der Pol et al. 2016). However, the challenges have been recognized and respective international organizations are committed to continuous standardization (Witwer et al. 2013). Novel, inexpensive and critically scrutinized methods are being developed in order to avoid the gaps of the earlier ones and to match expectations, for example, for personalized markers.
Whereas the physical properties and protein and RNA content of the various EV types significantly overlap, a careful selection of methods available should provide a rational basis for an appropriate selection of each type of application (Musante et al. 2014b; Rupert et al. 2017).
Notably, methods for single vesicle detection are developing rapidly, including those utilizing microfluidics and nano-sized beads. These will be highly useful, especially for linking the different vesicle types to specific contents and, thus, for revealing details of their functions. The respective compact systems and techniques including micro-nuclear magnetic resonanace (Shao et al. 2012), magneto-electrochemical sensor systems (Jeong et al. 2016) and nanoplasmonic chips (Im et al. 2014) are expected to develop into practical and rapid point-of-care detection for diagnostics, especially for uEV-based protein miRNA biomarkers (Shao et al. 2015). Flow cytometry is currently developing as an exciting extension for EV isolation directly in suspension and provides a means for the efficient identification of EV based on their type-specific epitopes and sequestration into subpopulations. However, currently, the size-range of flow cytometric detection starts at 200 nm going upwards, although a detection limit down to 100 nm has been achieved (Pospichalova et al. 2015; Rupert et al. 2017). Thus, FACS detection, especially of exosomes, is currently beyond the detection limit (Shao et al. 2015).
The massively parallel Next-Generation Sequencing as applied also to uEV analytics is a welcome extension to the repertoire of the novel robust and non-biased methods for urinary analytics (Renkema et al. 2014; Khurana et al. 2017). Carefully planned studies, meticulous controls and critical evaluation of results will provide an unforeseen accuracy to mechanisms of health and disease beyond the kidney parenchyme and urinary tract. The obvious caveat of genetic material as a “contaminant” from bacterial OMVs should be noted. Moreover, novel mechanisms to distinguish bacterial from “non-bacterial” cystitis, an important clinical problem, are expected to be discovered with emerging new therapeutic options.
An exciting extension to utilizing EV biology relates to bioengineered nanoparticles. These can serve as EV mimics and can be released into the circulation with a defined target cell “address code”. Upon attachment, the engineered protein, regulatory RNA or selected medication contents can be released as previously exemplified by the delivery of targeted chemotherapeutics (Jang et al. 2013). These treatments will have a huge influence, including on kidney diseases, with next-generation targeted therapies fully utilizing the obtained knowledge of EV biology and their bioengineering capacities.
References
Akers JC, Gonda D, Kim R, Carter BS, Chen CC (2013) Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neuro-Oncol 113:1–11
Alter ML, Kretschmer A, Von Websky K, Tsuprykov O, Reichetzeder C, Simon A, Stasch JP, Hocher B (2012) Early urinary and plasma biomarkers for experimental diabetic nephropathy. Clin Lab 58:659–671
Alvarez ML, Distefano JK (2013) The role of non-coding RNAs in diabetic nephropathy: potential applications as biomarkers for disease development and progression. Diabetes Res Clin Pract 99:1–11
Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK (2012) Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int 82:1024–1032
Alvarez S, Suazo C, Boltansky A, Ursu M, Carvajal D, Innocenti G, Vukusich A, Hurtado M, Villanueva S, Carreno JE, Rogelio A, Irarrazabal CE (2013) Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant Proc 45:3719–3723
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
Argyropoulos C, Wang K, Bernardo J, Ellis D, Orchard T, Galas D, Johnson JP (2015) Urinary microRNA profiling predicts the development of microalbuminuria in patients with type 1 diabetes. J Clin Med 4:1498–1517
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 108:5003–5008
Bai J, Kim SI, Ryu S, Yoon H (2014) Identification and characterization of outer membrane vesicle-associated proteins in Salmonella enterica serovar typhimurium. Infect Immun 82:4001–4010
Barutta F, Tricarico M, Corbelli A, Annaratone L, Pinach S, Grimaldi S, Bruno G, Cimino D, Taverna D, Deregibus MC, Rastaldi MP, Perin PC, Gruden G (2013) Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One 8:e73798
Bellingham SA, Guo BB, Coleman BM, Hill AF (2012) Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol 3:124
Brown L, Wolf JM, Prados-Rosales R, Casadevall A (2015) Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13:620–630
Brubaker L, Wolfe AJ (2015) The new world of the urinary microbiota in women. Am J Obstet Gynecol 213:644–649
Canfran-Duque A, Ramirez CM, Goedeke L, Lin CS, Fernandez-Hernando C (2014) microRNAs and HDL life cycle. Cardiovasc Res 103:414–422
Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF (2014) Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int 86:433–444
Cheung KH, Keerthikumar S, Roncaglia P, Subramanian SL, Roth ME, Samuel M, Anand S, Gangoda L, Gould S, Alexander R, Galas D, Gerstein MB, Hill AF, Kitchen RR, Lotvall J, Patel T, Procaccini DC, Quesenberry P, Rozowsky J, Raffai RL, Shypitsyna A, Su AI, Thery C, Vickers K, Wauben MH, Mathivanan S, Milosavljevic A, Laurent LC (2016) Extending gene ontology in the context of extracellular RNA and vesicle communication. J Biomed Semantics 7:19
Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P, Minciacchi VR, Di Vizio D (2016) Focus on extracellular vesicles: new frontiers of cell-to-cell communication in cancer. Int J Mol Sci 17:175
Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, Buzas EI, Lotvall J (2013) Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2:20677
Cvjetkovic A, Lotvall J, Lasser C (2014) The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles 3:23111
DeClerck YA, Mercurio AM, Stack MS, Chapman HA, Zutter MM, Muschel RJ, Raz A, Matrisian LM, Sloane BF, Noel A, Hendrix MJ, Coussens L, Padarathsingh M (2004) Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol 164:1131–1139
Delic D, Eisele C, Schmid R, Baum P, Wiech F, Gerl M, Zimdahl H, Pullen SS, Urquhart R (2016) Urinary exosomal miRNA signature in type II diabetic nephropathy patients. PLoS One 11:e0150154
El Andaloussi S, Mager I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12:347–357
El Nahas M (2005) The global challenge of chronic kidney disease. Kidney Int 68:2918–2929
Erdbrugger U, Le TH (2016) Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 27:12–26
Fernandez-Llama P, Khositseth S, Gonzales PA, Star RA, Pisitkun T, Knepper MA (2010) Tamm-Horsfall protein and urinary exosome isolation. Kidney Int 77:736–742
Gamez-Valero A, Lozano-Ramos SI, Bancu I, Lauzurica-Valdemoros R, Borras FE (2015) Urinary extracellular vesicles as source of biomarkers in kidney diseases. Front Immunol 6:6
Garcia-Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard H, Hansen T, Beckmann M, Pedersen O, Elliott P, Stamler J, Nicholson JK, Draper J, Mathers JC, Holmes E, Frost G (2017) Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol 5:184–195
Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF (2016) Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles 5:32945
Ghosh A, Davey M, Chute IC, Griffiths SG, Lewis S, Chacko S, Barnett D, Crapoulet N, Fournier S, Joy A, Caissie MC, Ferguson AD, Daigle M, Meli MV, Lewis SM, Ouellette RJ (2014) Rapid isolation of extracellular vesicles from cell culture and biological fluids using a synthetic peptide with specific affinity for heat shock proteins. PLoS One 9:e110443
Grosshans H, Filipowicz W (2008) Molecular biology: the expanding world of small RNAs. Nature 451:414–416
Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, Lee YC, Seol SY, Cho DT, Kim SI, Lee JC (2011) Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS One 6:e27958
Hildonen S, Skarpen E, Halvorsen TG, Reubsaet L (2016) Isolation and mass spectrometry analysis of urinary extraexosomal proteins. Sci Rep 6:36331
Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, Lee H (2014) Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol 32:490–495
Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS (2013) Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 7:7698–7710
Jeong S, Park J, Pathania D, Castro CM, Weissleder R, Lee H (2016) Integrated magneto-electrochemical sensor for exosome analysis. ACS Nano 10:1802–1809
Khurana R, Ranches G, Schafferer S, Lukasser M, Rudnicki M, Mayer G, Huttenhofer A (2017) Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA (New York) 23:142–152
Kim JH, Lee J, Park J, Gho YS (2015) Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol 40:97–104
Koeppen K, Hampton TH, Jarek M, Scharfe M, Gerber SA, Mielcarz DW, Demers EG, Dolben EL, Hammond JH, Hogan DA, Stanton BA (2016) A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog 12:e1005672
Korhonen TK, Virkola R, Westerlund B, Tarkkanen AM, Lahteenmaki K, Sareneva T, Parkkinen J, Kuusela P, Holthofer H (1988) Tissue interactions of Escherichia coli adhesins. Antonie Van Leeuwenhoek 54:411–420
Lane RE, Korbie D, Anderson W, Vaidyanathan R, Trau M (2015) Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep 5:7639
Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, Park KS, Lee JO, Kim YK, Kwon KH, Kim KP, Gho YS (2007) Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7:3143–3153
Lee LW, Zhang S, Etheridge A, Ma L, Martin D, Galas D, Wang K (2010) Complexity of the microRNA repertoire revealed by next-generation sequencing. RNA (New York) 16:2170–2180
Liu X, Chinello C, Musante L, Cazzaniga M, Tataruch D, Calzaferri G, James Smith A, De Sio G, Magni F, Zou H, Holthofer H (2015) Intraluminal proteome and peptidome of human urinary extracellular vesicles. Proteomics Clin Appl 9:568–573
Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM (2015) Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep 5:17319
Lunavat TR, Cheng L, Kim DK, Bhadury J, Jang SC, Lasser C, Sharples RA, Lopez MD, Nilsson J, Gho YS, Hill AF, Lotvall J (2015) Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells—evidence of unique microRNA cargos. RNA Biol 12:810–823
Ma L, Zhang XQ, Zhou DX, Cui Y, Deng LL, Yang T, Shao Y, Ding M (2016) Feasibility of urinary microRNA profiling detection in intrahepatic cholestasis of pregnancy and its potential as a non-invasive biomarker. Sci Rep 6:31535
Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF (2016) Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol 12:768–776
Mansueto G, Armani A, Viscomi C, D’Orsi L, De Cegli R, Polishchuk EV, Lamperti C, Di Meo I, Romanello V, Marchet S, Saha PK, Zong H, Blaauw B, Solagna F, Tezze C, Grumati P, Bonaldo P, Pessin JE, Zeviani M, Sandri M, Ballabio A (2017) Transcription factor EB controls metabolic flexibility during exercise. Cell Metab 25:182–196
Maughan RJ (1991) Fluid and electrolyte loss and replacement in exercise. J Sports Sci 9:117–142
Miranda KC, Bond DT, McKee M, Skog J, Paunescu TG, Da Silva N, Brown D, Russo LM (2010) Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int 78:191–199
Morrison EE, Bailey MA, Dear JW (2016) Renal extracellular vesicles: from physiology to clinical application. J Physiol (Lond) 594:5735–5748
Mulcahy LA, Pink RC, Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3:24641
Musante L, Saraswat M, Duriez E, Byrne B, Ravida A, Domon B, Holthofer H (2012) Biochemical and physical characterisation of urinary nanovesicles following CHAPS treatment. PLoS One 7:e37279
Musante L, Tataruch D, Gu D, Benito-Martin A, Calzaferri G, Aherne S, Holthofer H (2014a) A simplified method to recover urinary vesicles for clinical applications, and sample banking. Sci Rep 4:7532
Musante L, Tataruch DE, Holthofer H (2014b) Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Front Endocrinol (Lausanne) 5:149
Musante L, Tataruch D, Gu D, Liu X, Forsblom C, Groop PH, Holthofer H (2015) Proteases and protease inhibitors of urinary extracellular vesicles in diabetic nephropathy. J Diabetes Res 2015:289734
Musante L, Tataruch-Weinert D, Kerjaschki D, Henry M, Meleady P, Holthofer H (2017) Residual urinary extracellular vesicles in ultracentrifugation supernatants after hydrostatic filtration dialysis enrichment. J Extracell Vesicles 6:1267896
Nour AM, Modis Y (2014) Endosomal vesicles as vehicles for viral genomes. Trends Cell Biol 24:449–454
Patrakka J, Tryggvason K (2010) Molecular make-up of the glomerular filtration barrier. Biochem Biophys Res Commun 396:164–169
Patton JG, Franklin JL, Weaver AM, Vickers K, Zhang B, Coffey RJ, Ansel KM, Blelloch R, Goga A, Huang B, L’Etoille N, Raffai RL, Lai CP, Krichevsky AM, Mateescu B, Greiner VJ, Hunter C, Voinnet O, McManus MT (2015) Biogenesis, delivery, and function of extracellular RNA. J Extracell Vesicles 4:27494
Pazourkova E, Pospisilova S, Svobodova I, Horinek A, Brisuda A, Soukup V, Hrbacek J, Capoun O, Mares J, Hanus T, Babjuk M, Korabecna M (2016) Comparison of microRNA content in plasma and urine indicates the existence of a transrenal passage of selected microRNAs. Adv Exp Med Biol 924:97–100
Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101:13368–13373
Pollak MR, Quaggin SE, Hoenig MP, Dworkin LD (2014) The glomerulus: the sphere of influence. Clin J Am Soc Nephrol 9:1461–1469
Pospichalova V, Svoboda J, Dave Z, Kotrbova A, Kaiser K, Klemova D, Ilkovics L, Hampl A, Crha I, Jandakova E, Minar L, Weinberger V, Bryja V (2015) Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles 4:25530
Prud’homme GJ, Glinka Y, Lichner Z, Yousef GM (2016) Neuropilin-1 is a receptor for extracellular miRNA and AGO2/miRNA complexes and mediates the internalization of miRNAs that modulate cell function. Oncotarget 7:68057–68071
Ramezani A, Devaney JM, Cohen S, Wing MR, Scott R, Knoblach S, Singhal R, Howard L, Kopp JB, Raj DS (2015) Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur J Clin Invest 45:394–404
Remuzzi G, Benigni A, Remuzzi A (2006) Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116:288–296
Renkema KY, Stokman MF, Giles RH, Knoers NV (2014) Next-generation sequencing for research and diagnostics in kidney disease. Nat Rev Nephrol 10:433–444
Ricard-Blum S, Vallet SD (2016) Proteases decode the extracellular matrix cryptome. Biochimie 122:300–313
Rupert DL, Claudio V, Lasser C, Bally M (2017) Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim Biophys Acta 1861:3164–3179
Salih M, Zietse R, Hoorn EJ (2014) Urinary extracellular vesicles and the kidney: biomarkers and beyond. Am J Physiol Renal Physiol 306:F1251–F1259
Saritas T, Kuppe C, Moeller MJ (2015) Progress and controversies in unraveling the glomerular filtration mechanism. Curr Opin Nephrol Hypertens 24:208–216
Scott RP, Quaggin SE (2015) Review series: the cell biology of renal filtration. J Cell Biol 209:199–210
Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H (2012) Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med 18:1835–1840
Shao H, Chung J, Lee K, Balaj L, Min C, Carter BS, Hochberg FH, Breakefield XO, Lee H, Weissleder R (2015) Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun 6:6999
Shmaryahu A, Carrasco M, Valenzuela PD (2014) Prediction of bacterial microRNAs and possible targets in human cell transcriptome. J Microbiol (Seoul, Korea) 52:482–489
Sohel MH (2016) Extracellular/circulating microRNAs: release mechanisms, functions and challenges. Achievem Life Sci 10:175–186
Sorrentino S (1998) Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell Mol Life Sci 54:785–794
Tataruch-Weinert D, Musante L, Kretz O, Holthofer H (2016) Urinary extracellular vesicles for RNA extraction: optimization of a protocol devoid of prokaryote contamination. J Extracell Vesicles 5:30281
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
Tomasetti M, Lee W, Santarelli L, Neuzil J (2017) Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med 49:e285
Turco AE, Lam W, Rule AD, Denic A, Lieske JC, Miller VM, Larson JJ, Kremers WK, Jayachandran M (2016) Specific renal parenchymal-derived urinary extracellular vesicles identify age-associated structural changes in living donor kidneys. J Extracell Vesicles 5:29642
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
van Balkom BW, Pisitkun T, Verhaar MC, Knepper MA (2011) Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int 80:1138–1145
van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64:676–705
van der Pol E, Boing AN, Gool EL, Nieuwland R (2016) Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost 14:48–56
Van Roosbroeck K, Pollet J, Calin GA (2013) miRNAs and long noncoding RNAs as biomarkers in human diseases. Expert Rev Mol Diagn 13:183–204
Wachalska M, Koppers-Lalic D, van Eijndhoven M, Pegtel M, Geldof AA, Lipinska AD, van Moorselaar RJ, Bijnsdorp IV (2016) Protein complexes in urine interfere with extracellular vesicle biomarker studies. J Circ Biomark 5:4. DOI: 10.5772/62579
Wang D, Sun W (2014) Urinary extracellular microvesicles: isolation methods and prospects for urinary proteome. Proteomics 14:1922–1932
Wang K, Li H, Yuan Y, Etheridge A, Zhou Y, Huang D, Wilmes P, Galas D (2012) The complex exogenous RNA spectra in human plasma: an interface with human gut biota? PLoS One 7:e51009
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63
Watanabe K (2016) Bacterial membrane vesicles (MVs): novel tools as nature- and nano-carriers for immunogenic antigen, enzyme support, and drug delivery. Appl Microbiol Biotechnol 100:9837–9843
Willms E, Johansson HJ, Mager I, Lee Y, Blomberg KE, Sadik M, Alaarg A, Smith CI, Lehtio J, El Andaloussi S, Wood MJ, Vader P (2016) Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 6:22519
Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2:20360
Wolf P (1967) The nature and significance of platelet products in human plasma. Br J Haematol 13:269–288
Xu L, Yang BF, Ai J (2013) MicroRNA transport: a new way in cell communication. J Cell Physiol 228:1713–1719
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066
Yang Y, Xiao L, Li J, Kanwar YS, Liu F, Sun L (2013) Urine miRNAs: potential biomarkers for monitoring progression of early stages of diabetic nephropathy. Med Hypotheses 81:274–278
Zaborowski MP, Balaj L, Breakefield XO, Lai CP (2015) Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience 65:783–797
Acknowledgements
This study was supported by the EU-Innovative Medicines Initiative (IMI) Program “Biomarker Enterprise to Attack Diabetic Kidney Disease” (BEAt-DKD, Grant number 115974) and the FRIAS program of the Albert-Ludwigs University, Freiburg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Barreiro, K., Holthofer, H. Urinary extracellular vesicles. A promising shortcut to novel biomarker discoveries. Cell Tissue Res 369, 217–227 (2017). https://doi.org/10.1007/s00441-017-2621-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2621-0