Abstract
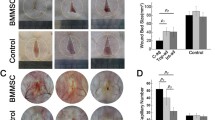
A new concept for wound therapy is the initiation of the regeneration of epidermal and dermal layers with appendages for skin function recovery. Bone-marrow-derived mesenchymal and epidermal stem cells (BMSCs and SSCs) are hypothesized to be able to home toward or to be transplanted to wound sites for skin repair and regeneration, but this awaits confirmation by further experimental and clinical evidence. In this study, the influence of the transplantation of BMSCs and SSCs with porous gelatin-β-tricalcium phosphate sponge as scaffolds on wound re-epithelization, collagen synthesis, skin tensile strength recovery, and skin appendage regeneration has been investigated. The transplantation of BMSCs or SSCs significantly accelerates wound re-epithelization, stimulates dermal collagen synthesis, and exhibits the trend to enhance the tensile strength recovery of skin. Furthermore, regenerative features of BMSCs and SSCs have been identified in activating blood vessel and hair follicle formation, respectively. These results not only provide experimental evidence for the application of BMSCs and SSCs as promising therapeutics for clinical wound treatment, but also display their characteristics in activating distinct skin appendage regeneration, which might have novel applications in skin tissue engineering.







Similar content being viewed by others
References
Anthony DM, Mark WJ (2007) Tissue engineering of replacement skin, the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4:413–437
Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L et al (2010) Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant 16:838–847
Barrandon Y (2007) Genetic manipulation of skin stem cells, success, hope, and challenges ahead. Mol Ther 15:443–444
Cristofanilli M, Harris VK, Zigelbaum A, Goossens AM, Lu A, Rosenthal H et al (2011) Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells Dev 20:2065–2076
Diegelmann RF, Evans MC (2004) Wound healing, an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289
Dorsett-Martin WA (2004) Rat models of skin wound healing, a review. Wound Repair Regen 12:591–599
Elizabeth M, Winter MD, Angelique AM, Oorschot V, Hogers B, Graaf VD et al (2009) A new direction for cardiac regeneration therapy application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail 2:643–653
Fu X, Li H (2009) Mesenchymal stem cells and skin wound repair and regeneration, possibilities and questions. Cell Tissue Res 335:317–321
Herzog EL, Chai L, Krause DS (2003) Plasticity of marrow-derived stem cells. Blood 102:3483–3493
Hoang MP, Keady M, Mahalingam M (2009) Stem cell markers (cytokeratin 15, CD34 and nestin) in primary scarring and nonscarring alopecia. Br J Dermatol 160:609–615
Huang E, Lian X, Chen W, Yang T, Yang L (2009) Characterization of rat hair follicle stem cells selected by vario magnetic activated cell sorting system. Acta Histochem Cytochem 42:129–136
Huang S, Lu G, Wu Y, Jirigala E, Xu YG, Ma K et al (2012) Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci 66:29–36
Jiang S, Zhao L, Purandare B, Hantash BM (2010) Differential expression of stem cell markers in human follicular bulge and interfollicular epidermal compartments. Histochem Cell Biol 133:455–465
Jin SO, Keung NK, Sung SA, Pennant WA, Kim HJ, Gwak SJ, Yoon do H, Lim MH, Choi BH, Ha Y (2011) Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transplant 20:837–849
Kai CW, Helmut D (2005) Mesenchymal stem cells for myocardial infarction. Circulation 112:151–153
Kamstrup M, Faurschou A, Gniadecki R, Wulf HC (2008) Epidermal stem cells—role in normal, wounded and pathological psoriatic and cancer skin. Curr Stem Cell Res 3:146–150
Kim DS, Cho HJ, Choi HR, Kwon SB, Park KC (2004) Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell Mol Life Sci 61:2774–2781
Kunz P, Hoffend J, Altmann A, Dimitrakopoulou-Strauss A, Koczan D (2006) Angiopoietin-2 overexpression in Morris hepatoma results in increased tumor perfusion and induction of critical angiogenesis-promoting genes. J Nucl Med 47:1515–1524
Lau K, Paus R, Tiede S, Day P, Bayat A (2009) Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol 18:921–933
Lauto A, Hook J, Doran M, Camacho F, Poole-Warren LA (2005) Chitosan adhesive for laser tissue repair: in vitro characterization. Lasers Surg Med 36:193–201
Lee KB, Choi J, Cho SB, Chung JY, Moon ES, Kim NS, Han HJ (2011) Topical embryonic stem cells enhance wound healing in diabetic rats. J Orthop Res 29:1554–1562
Liu K, Liu R, Cao G, Sun H, Wang X, Wu S (2011) Adipose-derived stromal cell autologous transplantation ameliorates pulmonary arterial hypertension induced by shunt flow in rat models. Stem Cells Dev 20:1001–1010
Liu Y, Zhou H, Gao F (2008) Isolation and identification of stem cells from adult cashmere goat skin. Int J Dermatol 47:551–556
Ma K, Liao S, He LM, Lu J, Ramakrishna S, Chan CK (2011) Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng Part A 17:1413–1424
Mansbridge JN (2009) Tissue-engineered skin substitutes in regenerative medicine. Curr Opin Biotechnol 20:563–567
Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science 276:75–81
Morasso MI, Tomic-Canic M (2005) Epidermal stem cells, the cradle of epidermal determination, differentiation and wound healing. Biol Cell 97:173–183
Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J et al (2008) The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 22:593–599
Nowak JA, Fuchs E (2009) Isolation and culture of epithelial stem cells. Methods Mol Biol 482:215–232
Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB et al (2006) Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest 116:249–260
Pellegrini G, Dellambra E, Golisano O, Martinelli E (2011) p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA 98:3156–3161
Peng LH, Fung KP, Leung PC, Gao JQ (2011) Genetically manipulated adult stem cells for wound healing. Drug Discov Today 16:956–966
Porada CD, Zanjani ED, Almeida-Porad G (2006) Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther 1:365–369
Pritinder K (2006) Interfollicular epidermal stem cells, identification, challenges, potential. J Invest Dermatol 126:1450–1458
Puymirat E, Geha R, Tomescot A, Bellamy V, Larghero J, Trinquart L, Bruneval P, Desnos M, Hagège A, Pucéat M, Menasché P (2009) Can mesenchymal stem cells induce tolerance to cotransplanted human embryonic stem cells? Mol Ther 17:176–182
Reiisi S, Esmaeili F, Shirazi A (2010) Isolation, culture and identification of epidermal stem cells from newborn mouse skin. In Vitro Cell Dev Biol Anim 46:54–59
Takahashi Y, Tabata Y (2003) Homogeneous seeding of mesenchymal stem cells into nonwoven fabric for tissue engineering. Tissue Eng 9:931–938
Takahashi Y, Yamamotom M, Tabata Y (2005a) Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials 26:4856–4865
Takahashi Y, Yamamoto M, Tabata Y (2005b) Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and b-tricalcium phosphate. Biomaterials 26:3587–3596
Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH (2010) Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg 65:565–572
Temple S (2003) Stem cell plasticity—building the brain of our dreams. Nat Rev 2:513–520
Velnar T, Bailey T, Smrkolj V (2009) The wound healing process, an overview of the cellular and molecular mechanisms. J Int Med Res 37:1528–1542
Wang G, Ao Q, Gong K, Zuo HC, Gong Y, Zhang XF (2010) Synergistic effect of neural stem cells and olfactory ensheathing cells on repair of adult rat spinal cord injury. Cell Transplant 19:1325–1337
Wang W, Lin S, Xiao Y, Huang Y (2008) Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci 82:190–204
Wang Y, Nathanson L, McNiece IK (2011) Differential hematopoietic supportive potential and gene expression of stroma cell lines from midgestation mouse placenta and adult bone marrow. Cell Transplant 20:707–726
Wang Z, Gao Z, Shi Y, Sun Y (2007) Inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J Plast Reconstr Aesthetic Surg 60:1193–1199
Webb A, Li A, Kaur P (2004) Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 72:387–395
Weinand C, Nabili A, Khumar M, Dunn JR, Ramella-Roman J, Jeng JC, Jordan MH, Tabata Y (2010) Factors of osteogenesis influencing various human stem cells on third-generation gelatin/β-tricalcium phosphate scaffold material. Rejuvenation Res 14:185–194
Wu J, Sun Z, Sun HS, Wu J, Weisel RD, Keating A et al (2008) Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant 16:993–1005
Yang HM, Cho MR, Sung JH, Yang SJ, Nam MH, Roh CR, Kim JM, Shin M, Song SH, Kwon CH, Joh JW, Kim SJ (2011) The effect of human fetal liver-derived mesenchymal stem cells on CD34+ hematopoietic stem cell repopulation in NOD/Shi-scid/IL-2Rã(null) mice. Transplant Proc 43:2004–2008
Young KJ, Jang YH, Yoo DR, Kim SN, Lee SK, Nam MJ (2010) Mesenchymal stem cells’ interaction with skin: wound-healing effect on fibroblast cells and skin tissue. Wound Rep Reg 18:655–661
Zhao Y, Zhang Z, Wang J, Yin P, Zhou J, Zhen M, Cui W, Xu G, Yang D, Liu Z (2012) Abdominal hernia repair with a decellularized dermal scaffold seeded with autologous bone-marrow-derived mesenchymal stem cells. Artif Organs 36:247–255
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the National Natural Science Foundation of China (Grants No: 81102393, 30873173), the 48th China Postdoctoral Science Foundation (Grant No: 420000-X91004), the Fundamental Research Funds for the Central Universities, China, the the Basic Research Funds for the Zhejiang University and the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
Rights and permissions
About this article
Cite this article
Peng, LH., Mao, ZY., Qi, XT. et al. Transplantation of bone-marrow-derived mesenchymal and epidermal stem cells contribute to wound healing with different regenerative features. Cell Tissue Res 352, 573–583 (2013). https://doi.org/10.1007/s00441-013-1609-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1609-7