Abstract
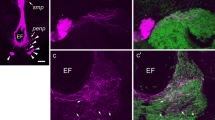
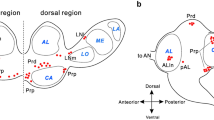
A few types of peptidergic clock neurons have been identified in the fruitfly Drosophila, whereas in blowflies, only pigment-dispersing factor (PDF)-immunoreactive lateral ventral clock neurons (LNvs) have been described. In blowflies, but not Drosophila, a subset of these PDF-expressing neurons supplies axon branches to a region outside the synaptic layer of the lamina, the most peripheral optic lobe neuropil. In Drosophila, similar lamina processes are instead supplied by non-clock neurons (LMIo) that express myoinhibitory peptide (MIP). We have investigated the distribution of MIP-immunoreactive neurons in the visual system of the blowfly Calliphora vomitoria and found neurons resembling the three LMIos, but without processes to the lamina. In Calliphora, PDF-immunoreactive processes of LNvs in the lamina closely impinge on branching serotonin-immunoreactive axon terminations in the same region. We have also identified, in the blowfly, two types of putative clock neurons that label with an antiserum to ion-transport peptide (ITP). The presence of serotonin-immunoreactive neurons supplying processes to the lamina seems to be a conserved feature in dipteran flies. The morphology of the two types of ITP-immunoreactive clock neurons might also be conserved. However, peptidergic neurons with branches converging on the serotonin-immunoreactive neurons in the lamina are of different morphological types and express PDF in blowflies and MIP in Drosophila. The central circuitry of these PDF- and MIP-expressing neurons probably differs; consequently, whether their convergence on serotonergic neurons subserves similar functions in the two species is unclear.







Similar content being viewed by others
References
Carlsson MA, Diesner M, Schachtner J, Nässel DR (2010) Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J Comp Neurol 518:3359–3380
Chen B, Meinertzhagen IA, Shaw SR (1999) Circadian rhythms in light-evoked responses of the fly's compound eye, and the effects of neuromodulators 5-HT and the peptide PDF. J Comp Physiol A Sens Neural Behav Physiol 185:393–404
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP (1987) The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: identification by an antiserum against a synthetic PDH. Cell Tissue Res 250:377–387
Dircksen H, Tesfai LK, Albus C, Nässel DR (2008) Ion transport peptide splice forms in central and peripheral neurons throughout postembryogenesis of Drosophila melanogaster. J Comp Neurol 509:23–41
Helfrich-Förster C (1995) The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci USA 92:612–616
Helfrich-Förster C, Homberg U (1993) Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol 337:177–190
Helfrich-Förster C, Shafer OT, Wulbeck C, Grieshaber E, Rieger D, Taghert P (2007a) Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol 500:47–70
Helfrich-Förster C, Yoshii T, Wulbeck C, Grieshaber E, Rieger D, Bachleitner W, Cusamano P, Rouyer F (2007b) The lateral and dorsal neurons of Drosophila melanogaster: new insights about their morphology and function. Cold Spring Harb Symp Quant Biol 72:517–525
Hevers W, Hardie RC (1995) Serotonin modulates the voltage dependence of delayed rectifier and Shaker potassium channels in Drosophila photoreceptors. Neuron 14:845–856
Homberg U (2002) Neurotransmitters and neuropeptides in the brain of the locust. Microsc Res Tech 56:189–209
Homberg U, Brandl C, Clynen E, Schoofs L, Veenstra JA (2004) Mas-allatotropin/Lom-AG-myotropin I immunostaining in the brain of the locust, Schistocerca gregaria. Cell Tissue Res 318:439–457
Johard HA, Yoishii T, Dircksen H, Cusumano P, Rouyer F, Helfrich-Förster C, Nässel DR (2009) Peptidergic clock neurons in Drosophila: ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J Comp Neurol 516:59–73
Kahsai L, Winther AM (2010) Chemical neuroanatomy of the Drosophila central complex: distribution of multiple neuropeptides in relation to neurotransmitters. J Comp Neurol 519:290–315
Kim YJ, Bartalska K, Audsley N, Yamanaka N, Yapici N, Lee JY, Kim YC, Markovic M, Isaac E, Tanaka Y, Dickson BJ (2010) MIPs are ancestral ligands for the sex peptide receptor. Proc Natl Acad Sci USA 107:6520–6525
Kolodziejczyk A, Nässel DR (2011) A novel wide-field neuron with branches in the lamina of the Drosophila visual system expresses myoinhibitory peptide and may be associated with the clock. Cell Tissue Res 343:357–369
Muguruma F, Goto SG, Numata H, Shiga S (2010) Effect of photoperiod on clock gene expression and subcellular distribution of PERIOD in the circadian clock neurons of the blow fly Protophormia terraenovae. Cell Tissue Res 340:497–507
Nässel DR (1991) Neurotransmitters and neuromodulators in the insect visual system. Prog Neurobiol 37:179–254
Nässel DR (1999) Histamine in the brain of insects: a review. Microsc Res Tech 44:121–136
Nässel DR, Elekes K (1992) Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res 267:147–167
Nässel DR, Homberg U (2006) Neuropeptides in interneurons of the insect brain. Cell Tissue Res 326:1–24
Nässel DR, Meyer EP, Klemm N (1985) Mapping and ultrastructure of serotonin-immunoreactive neurons in the optic lobes of three insect species. J Comp Neurol 232:190–204
Nässel DR, Ohlsson L, Sivasubramanian P (1987) Postembryonic differentiation of serotonin-immunoreactive neurons in fleshfly optic lobes developing in situ or cultured in vivo without eye discs. J Comp Neurol 255:327–340
Nässel DR, Holmqvist MH, Hardie RC, Håkanson R, Sundler F (1988) Histamine-like immunoreactivity in photoreceptors of the compound eyes and ocelli of the flies Calliphora erythrocephala and Musca domestica. Cell Tissue Res 253:639–646
Nässel DR, Shiga S, Wikstrand EM, Rao KR (1991) Pigment-dispersing hormone-immunoreactive neurons and their relation to serotonergic neurons in the blowfly and cockroach visual system. Cell Tissue Res 266:511–523
Nässel DR, Shiga S, Mohrherr CJ, Rao KR (1993) Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. J Comp Neurol 331:183–198
Predel R, Rapus J, Eckert M (2001) Myoinhibitory neuropeptides in the American cockroach. Peptides 22:199–208
Pyza E, Meinertzhagen IA (1995) Monopolar cell axons in the first optic neuropil of the housefly, Musca domestica L., undergo daily fluctuations in diameter that have a circadian basis. J Neurosci 15:407–418
Pyza E, Meinertzhagen IA (1996) Neurotransmitters regulate rhythmic size changes amongst cells in the fly's optic lobe. J Comp Physiol 178:33–45
Pyza E, Siuta T, Tanimura T (2003) Development of PDF-immunoreactive cells, possible clock neurons, in the housefly Musca domestica. Microsc Res Tech 62:103–113
Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791–802
Ring M, Meredith J, Wiens C, Macins A, Brock HW, Phillips JE, Theilmann DA (1998) Expression of Schistocerca gregaria ion transport peptide (ITP) and its homologue (ITP-L) in a baculovirus/insect cell system. Insect Biochem Mol Biol 28:51–58
Santos JG, Vömel M, Struck R, Homberg U, Nässel DR, Wegener C (2007) Neuroarchitecture of peptidergic systems in the larval ventral ganglion of Drosophila melanogaster. PLoS ONE 2:e695
Shiga S, Numata H (2009) Roles of PER immunoreactive neurons in circadian rhythms and photoperiodism in the blow fly, Protophormia terraenovae. J Exp Biol 212:867–877
Sinakevitch I, Strausfeld NJ (2006) Comparison of octopamine-like immunoreactivity in the brains of the fruit fly and blow fly. J Comp Neurol 494:460–475
Sinakevitch I, Niwa M, Strausfeld NJ (2005) Octopamine-like immunoreactivity in the honey bee and cockroach: comparable organization in the brain and subesophageal ganglion. J Comp Neurol 488:233–254
Wendt B, Homberg U (1992) Immunocytochemistry of dopamine in the brain of the locust Schistocerca gregaria. J Comp Neurol 321:387–403
Yamanaka N, Hua YJ, Roller L, Spalovska-Valachova I, Mizoguchi A, Kataoka H, Tanaka Y (2010) Bombyx prothoracicostatic peptides activate the sex peptide receptor to regulate ecdysteroid biosynthesis. Proc Natl Acad Sci USA 107:2060–2065
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Supplementary material Fig. 1
Preabsorption test for myoinhibitory peptide (MIP) antiserum on cryostat sections of Calliphora (a–d) and Drosophila (e, f). After the MIP antiserum (αMIP) had been preabsorbed with 50 nM MIP-3 (presbsorbed), most immunolabeling was abolished (b, d, f). Control sections labeled with normal antiserum are shown in a, c, e (JPEG 129 kb)
Rights and permissions
About this article
Cite this article
Kolodziejczyk, A., Nässel, D.R. Myoinhibitory peptide (MIP) immunoreactivity in the visual system of the blowfly Calliphora vomitoria in relation to putative clock neurons and serotonergic neurons. Cell Tissue Res 345, 125–135 (2011). https://doi.org/10.1007/s00441-011-1198-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1198-2