Abstract
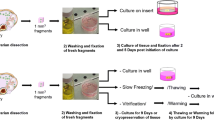
Caprine preantral follicles within ovarian fragments were exposed to or vitrified in the presence of sucrose, dimethyl sulfoxide (DMSO), ethylene glycol (EG), or various combinations thereof. The fragments were cryopreserved by using either a conventional (CV) or a solid-surface vitrification (SSV) protocol, and the cryoprotectants were removed by equilibrating vitrified ovarian fragments in “warming solution” consisting of minimum essential medium and heat-inactivated fetal calf serum (MEM+) followed by washes in MEM+ with or without sucrose. Histological analysis of follicle integrity showed that the percentages of normal follicles in ovarian fragments vitrified in sucrose mixed with EG and/or DMSO (CV method) or mixed with EG or DMSO (SSV method) followed by washes in MEM+ plus sucrose were similar to those of controls (ovarian fragments fixed without previous vitrification). Unlike for MEM+ (supplemented or unsupplemented by sucrose) and DMSO followed by washes in the absence of sucrose, the percentages of normal follicles found after exposure to cryoprotectant did not significantly differ from that found after vitrification, indicating that follicular degeneration was attributable to a toxic effect of cryoprotectants and not to the vitrification procedure. The viability of preantral follicles after the CV and SSV procedures was investigated by using calcein-AM and the ethidium-homodimer as “live” and “dead” markers, respectively. In both tested vitrification procedures, the highest percentages of viable follicles were observed when a mixture of sucrose and EG (70.3% for CV and 72.4% for SSV) was used. Preantral follicles were also vitrified (either by CV or SSV) in sucrose and EG and then cultured for 24 h, after which their viability was compared with that of cultured fresh and uncultured vitrified follicles. The viability of these follicles was maintained after SSV, but not after CV. Thus, the viability of caprine preantral follicles can be best preserved after SSV in a mixture of sucrose and EG, followed by washes in medium containing sucrose.


Similar content being viewed by others
References
Al-Aghbari AM, Menino AR (2002) Survival of oocytes recovered from vitrified sheep ovarian tissues. Anim Reprod Sci 71:101–110
Amorim CA, Rondina D, Rodrigues APR, Costa SHF, Goncalves PBD, Figueiredo JR, Giorgetti A (2003) Isolated ovine primodial follicles cryopreserved in different concentrations of ethylene glycol. Theriogenology 60:735–742
Bautista JA, Kanagawa H (1998) Current status of vitrification of embryos and oocytes in domestic animals: ethylene glycol as an emerging cryoprotectant of choice. Jpn J Vet Res 45:183–191
Begin I, Bhatia B, Baldassarre H, Dinnyes A, Keefer CL (2003) Cryopreservation of goat oocytes and in vivo derived 2- to 4-cell embryos using the cryoloop (CLV) and solid-surface vitrification (SSV) methods. Theriogenology 59:1839–1850
Chen ZJ, Li M, Zhao LX, Tang R, Sheng Y, Gao X, Chang CH, Feng HL (2004) Effects of sucrose concentration on the developmental potential of human frozen-thawed oocytes at different stages of maturity. Hum Reprod 19:2345–2349
Cortvrindt RG, Smitz JEJ (2001) Fluorescent probes allow rapid and precise recording of follicle density and staging in human ovarian cortical biopsy samples. Fertil Steril 75:588–593
Crowe JH, Carpenter JF, Crowe LM (1998) The role of vitrification in anhydrobiosis. Annu Rev Physiol 60:73–103
De Clerck LS, Bridts CH, Mertens AM, Moens MM, Stevens WJ (1994) Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. J Immunol Methods 172:115–124
Decherchi P, Cochard P, Gauthier P (1997) Dual staining assessment of Schwann cell viability within whole peripheral nerves using calcein-AM and ethidium homodimer. J Neurosci Methods 71:205–213
Demirci B, Lornage J, Salle B, Frappart L, Franck M, Guerin JF (2001) Follicular viability and morphology of sheep ovaries after exposure to cryoprotectant and cryopreservation with different freezing protocols. Fertil Steril 75:754–762
Dinnyes A, Dai Y, Jiang S, Yang X (2000) High developmental rates of vitrified bovine oocytes following parthenogenetic activation, and somatic cell nuclear transfer. Biol Reprod 63:513–518
Fabri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C (2001) Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod 16:411–416
Hochi S, Fujimoto T, Braun J, Oguri N (1994) Pregnancies following transfer of equine embryos cryopreserved by vitrification. Theriogenology 42:483–488
Hovatta O (2005) Methods for cryopreservation of human ovarian tissue. Reprod Biomed Online 10:729–734
Isachenko E, Isachenko V, Ramihi G, Nawroth F (2003) Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen. Eur J Obstet Gynecol Reprod Biol 108:186–193
Lucci CM, Amorim CA, Bao SN, Figueiredo JR, Rodrigues APR, Silva JRV, Goncalves PBD (1999) Effect of the interval of serial sections of ovarian tissue in the tissue chopper on the number of isolated caprine preantral follicles. Anim Reprod Sci 56:39–49
Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J (2004) Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril 82:1390–1394
Martino A, Pollard JW, Leibo SP (1996) Effect of chilling bovine oocytes on their developmental competence. Mol Reprod Dev 45:503–512
Naik BR, Rao BS, Vagdevi R, Gnanprakash M, Amarnath D, Rao VH (2005) Conventional slow freezing, vitrification and open pulled straw (OPS) vitrification of rabbit embryos. Anim Reprod Sci 86:329–338
Paynter SJ, Cooper A, Fuller BJ, Shaw RW (1999) Cryopreservation of bovine ovarian tissue: structural normality of follicles after thawing and culture in vitro. Cryobiology 38:301–309
Pena FJ, Saraiva F, Johannisson A, Walgren M, Rodriguez-Martinez H (2005) A new and simple method to evaluate early membrane changes in frozen boar spermatozoa. Int J Androl 28:107–114
Poole CA, Brookes NH, Clover GM (1993) Keratocyte networks visualized in the living cornea using vital dyes. J Cell Sci 106:685–692
Rahimi G, Isachenko E, Isachenko V, Sauer H, Wartenberg M, Tawadros S, Hescheler J, Mallmann P, Nawroth F (2004) Comparison of necrosis in human ovarian tissue after conventional slow freezing or vitrification and transplantation in ovariectomized SCID mice. Reprod Biomed Online 9:187–193
Rall WF, Fahy GM (1985) Ice-free cryopreservation of mouse embryos at −196 degrees C by vitrification. Nature 24:387–402
Rodrigues APR, Amorim CA, Costa SH, Matos MHT, Santos RR, Lucci CM, Bao SN, Ohashi OM, Figueiredo JR (2004a) Cryopreservation of caprine ovarian tissue using glycerol and ethylene glycol. Theriogenology 61:1009–1024
Rodrigues APR, Amorim CA, Costa SH, Matos MHT, Santos RR, Lucci CM, Bao SN, Ohashi OM, Figueiredo JR (2004b) Cryopreservation of caprine ovarian tissue using dimethylsulphoxide and propanediol. Anim Reprod Sci 84:211–227
Salehnia M (2002) Autograft of vitrified mouse ovaries using ethylene glycol as cryoprotectant. Exp Anim 51:509–512
Salehnia M, Abbasian ME, Rezazadeh VM (2002) Ultrastructure of follicles after vitrification of mouse ovarian tissue. Fertil Steril 78:644–645
Santos RR, Tharasanit T, Figueiredo JR, Van Haeften T, Van den Hurk R (2006) Improved preservation of caprine preantral follicle viability after cryopreservation in sucrose and ethylene glycol. Cell Tissue Res 325(3):523–531
Schotanus K, Hage WJ, Vanderstichele H, Van den Hurk R (1997) Effects of conditioned media from murine granulosa cell lines on the growth of isolated bovine preantral follicles. Theriogenology 48:471–483
Shaw J, Oranratnachai A, Trounson A (2000) Fundamental cryobiology of ammalian oocytes and ovarian tissue. Theriogenology 53:59–72
Stachecki JJ, Cohen J (2004) An overview of oocyte cryopreservation. Reprod Biomed Online 9:152–163
Toth TL, Baka SG, Veeck LL (1994) Fertilization and in vitro development of cryopreserved human prophase I oocytes. Fertil Steril 61:891–894
Vajta G, Booth PJ, Holm P, Greve T, Callesen H (1997) Successful vitrification of early stage bovine in vitro produced embryos with the open pulled straw (OPS) method. Cryo-letters 18:191–195
Van den Hurk R, Spek ER, Hage WJ, Fair T, Ralph JH, Schotanus K (1998) Ultrastructure and viability of isolated bovine preantral follicles. Hum Reprod Update 4:833–841
Vicente JS, Garcia-Ximenez F (1996) Direct transfer of vitrified embryos. Theriogenology 45:811–815
Wright DL, Eroglu A, Toner M, Toth TL (2004) Use of sugars in cryopreserving human oocytes. Reprod Biomed Online 9:179–186
Yeoman RR, Wolf DP, Lee DM (2005) Coculture of monkey ovarian tissue increases survival after vitrification and slow-rate freezing. Fertil Steril 83:1248–1254
Acknowledgements
The authors thank Peter Ursem, Arend Rijneveld, Ineke Daemen, Frans van Kooi, and the technicians of the Department of Pathobiology for their help.
Author information
Authors and Affiliations
Corresponding author
Additional information
CAPES/Brazil supported this work. Regiane Rodrigues dos Santos is a recipient of a grant from CAPES/Brazil.
Rights and permissions
About this article
Cite this article
Santos, R.R., Tharasanit, T., Van Haeften, T. et al. Vitrification of goat preantral follicles enclosed in ovarian tissue by using conventional and solid-surface vitrification methods. Cell Tissue Res 327, 167–176 (2007). https://doi.org/10.1007/s00441-006-0240-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0240-2