Abstract
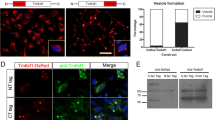
The effects of bafilomycin, nocodazole, and reduced temperature on recycling and the lysosomal pathway have been investigated in various cultured cell lines and have been shown to vary dependent on the cell type examined. However, the way in which these treatments affect recycling and transport to lysosomes within the same cell line has not been analyzed. In the current study, we used fluorophore-labeled transferrin and dextran as typical markers for the recycling and the lysosomal pathways, respectively, to explore the morphology and the intravesicular pH of endocytic compartments in HeLa cells. The V-ATPase inhibitor bafilomycin selectively inhibited the transport of marker destined for lysosomal degradation in early endosomes, whereas the transport of transferrin to the perinuclear recycling compartment (PNRC) still occurred. The kinetics of transferrin acidification was found to be biphasic, indicative of fast and slow recycling pathways via early endosomes (pH 6.0) and PNRC (pH 5.6), respectively. Furthermore, the disruption of microtubules by nocodazole blocked the transport of transferrin to the PNRC in early endosomes and of lysosome-directed marker into endosomal carrier vesicles. In contrast, incubation at 20°C affected the lysosomal pathway by causing retention of internalized dextran in late endosomes and a delay in transferrin recycling. Taken together, these data clearly demonstrate, for the first time, that the transferrin recycling pathway and transport of endocytosed material to lysosomes are differentially affected by bafilomycin, nocodazole, and low temperature in HeLa cells. Consequently, these treatments can be applied to investigate whether internalized macromolecules such as viruses follow a recycling or degradative pathway.











Similar content being viewed by others
References
Alami M, Taupiac MP, Reggio H, Bienvenue A, Beaumelle B (1998) Involvement of ATP-dependent Pseudomonas exotoxin translocation from a late recycling compartment in lymphocyte intoxication procedure. Mol Biol Cell 9:387–402
Aniento F, Gu F, Parton RG, Gruenberg J (1996) An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol 133:29–41
Apodaca G (2001) Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2:149–159
Barroso M, Sztul ES (1994) Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome. J Cell Biol 124:83–100
Bayer N, Schober D, Prchla E, Murphy RF, Blaas D, Fuchs R (1998) Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J Virol 72:9645–9655
Bowman E, Siebers A, Altendorf K (1988) Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA 85:7972–7976
Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB (1997) Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol 139:469–484
Cain CC, Sipe DM, Murphy RF (1989) Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc Natl Acad Sci USA 86:544–548
Chung J, Wessling-Resnick M (2003) Molecular mechanisms and regulation of iron transport. Crit Rev Clin Lab Sci 40:151–182
Clague MJ, Urbe S, Aniento F, Gruenberg J (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem 269:21–24
Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422:37–44
Cupers P, Veithen A, Kiss A, Baudhuin P, Courtoy PJ (1994) Clathrin polymerization is not required for bulk-phase endocytosis in rat fetal fibroblasts. J Cell Biol 127:725–735
Daro E, Sluijs P van der, Galli T, Mellman I (1996) Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA 93:9559–9564
Daro E, Sheff D, Gomez M, Kreis T, Mellman I (1997) Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J Cell Biol 139:1747–1759
D’Arrigo A, Bucci C, Toh BH, Stenmark H (1997) Microtubules are involved in bafilomycin A1-induced tubulation and Rab5-dependent vacuolation of early endosomes. Eur J Cell Biol 72:95–103
Deng YP, Storrie B (1988) Animal cell lysosomes rapidly exchange membrane proteins. Proc Nat Acad Sci USA 85:3860–3864
Deurs B van, Holm PK, L. K, Sandvig K (1995) Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosomes and preexisting lysosomes. Eur J Cell Biol 66:309–323
Dunn WA, Hubbard AL, Aronson NN (1980) Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem 255:5971–5978
Ellinger I, Klapper H, Fuchs R (1998) Fluid-phase marker transport in rat liver: free-flow electrophoresis separates distinct endosome subpopulations. Electrophoresis 19:1154–1161
Ellinger I, Klapper H, Courtoy PJ, Vaerman JP, Fuchs R (2002) Different temperature sensitivity of endosomes involved in transport to lysosomes and transcytosis in rat hepatocytes: analysis by free-flow electrophoresis. Electrophoresis 23:2117–2129
Geuze HJ, Slot JW, Schwartz AL (1987) Membranes of sorting organelles display lateral heterogeneity in receptor distribution. J Cell Biol 104:1715–1723
Ghosh RN, Gelman DL, Maxfield FR (1994) Quantification of low density lipoprotein and transferrin endocytic sorting HEp2 cells using confocal microscopy. J Cell Sci 107:2177–2189
Griffiths G, Back R, Marsh M (1989) A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J Cell Biol 109:2703–2720
Gruenberg J, Maxfield FR (1995) Membrane transport in the endocytic pathway. Curr Opin Cell Biol 7:552–563
Gruenberg J, Griffiths G, Howell K (1989) Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol 108:1301–1316
Harada M, Shakado S, Sakisaka S, Tamaki S, Ohishi M, Sasatomi K, Koga H, Sata M, Tanikawa K (1997) Bafilomycin A1, a specific inhibitor of V-type H+-ATPases, inhibits the acidification of endocytic structures and inhibits horseradish peroxidase uptake in isolated rat sinusoidal endothelial cells. Liver 17:244–250
Jing S, Spencer T, Miller K, Hopkins C, Trowbridge IS (1990) Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol 110:283–294
Johnson LS, Dunn KW, Pytowski B, McGraw TE (1993) Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor’s internalization motif. Mol Biol Cell 4:1251–1266
Killisch I, Steinlein P, Romisch K, Hollinshead R, Beug H, Griffiths G (1992) Characterization of early and late endocytic compartments of the transferrin cycle—transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J Cell Sci 103:211–232
Klapper H, Graf J, Fuchs R (1992) Temperature dependence of transcytotic pathways in rat liver. In: Courtoy PJ (ed) Endocytosis: from cell biology to health, disease and therapy, vol H62. Springer, Berlin Heidelberg New York, pp 301–307
Kronenberger P, Schober D, Prchla E, Blaas D, Fuchs R (1997) Use of free-flow electrophoresis for the analysis of cellular uptake of picornaviruses. Electrophoresis 18:2531–2536
Leung SM, Ruiz WG, Apodaca G (2000) Sorting of membrane and fluid at the apical pole of polarized Madin-Darby canine kidney cells. Mol Biol Cell 11:2131–2150
Lin SX, Gundersen GG, Maxfield FR (2002) Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol Biol Cell 13:96–109
Maxfield FR, McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Biol 5:121–132
Mayor S, Presley J, Maxfield F (1993) Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol 121:1257–1269
McGraw TE, Dunn KW, Maxfield FR (1993) Isolation of a temperature-sensitive variant Chinese hamster ovary cell line with a morphologically altered endocytic recycling compartment. J Cell Physiol 155:579–594
Mellman I, Fuchs R, Helenius A (1986) Acidification of the endocytic and exocytic pathways. Ann Rev Biochem 55:663–700
Miaczynska M, Zerial M (2002) Mosaic organization of the endocytic pathway. Exp Cell Res 272:8–14
Mueller SC, Hubbard AL (1986) Receptor-mediated endocytosis of asialoglycoproteins by rat hepatocytes: receptor-positive and receptor-negative endosomes. J Cell Biol 102:932–942
Mukherjee S, Ghosh RN, Maxfield FR (1997) Endocytosis. Physiol Rev 77:759–803
Murphy RF, Powers S, Cantor CR (1984) Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J Cell Biol 98:1757–1762
Nishi T, Forgac M (2002) The vacuolar (H+)-ATPases–nature’s most versatile proton pumps. Nat Rev Mol Cell Biol 3:94–103
Prchla E, Kuechler E, Blaas D, Fuchs R (1994) Uncoating of human rhinovirus serotype 2 from late endosomes. J Virol 68:3713–3723
Presley JF, Mayor S, McGraw TE, Dunn KW, Maxfield FR (1997) Bafilomycin A1 treatment retards transferrin receptor recycling more than bulk membrane recycling. J Biol Chem. 272:13929–13936
Renwoude J van, Bridges K, Harford J, Klausner R (1982) Receptor-mediated endocytosis of transferrin and the uptake of Fe in K562 cells: identification of a non-lysosomal acidic compartment. Proc Natl Acad Sci USA 79:6186–6190
Roederer M, Bowser R, Murphy RF (1987) Kinetics and temperature dependence of exposure of endocytosed material to proteolytic enzymes and low pH: evidence for a maturation model for the formation of lysosomes. J Cell Physiol 131:200–209
Runnegar M, Wei X, Berndt N, Hamm-Alvarez S (1997) Transferrin receptor recycling in rat hepatocytes is regulated by protein phosphatase 2A, possibly through effects on microtubule-dependent transport. Hepatology 26:176–185
Rybak S, Murphy R (1998) Primary cell cultures from murine kidney and heart differ in endosomal pH. J Cell Physiol 176:216–222
Schober D, Kronenberger P, Prchla E, Blaas D, Fuchs R (1998) Major and minor receptor group human rhinoviruses penetrate from endosomes by different mechanisms. J Virol 72:1354–1364
Sheff DR, Daro EA, Hull M, Mellman I (1999) The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol 145:123–139
Sipe DM, Murphy RF (1987) High-resolution kinetics of transferrin acidification in BALB/c 3T3 cells: exposure to pH 6 followed by temperature-sensitive alkalinization during recycling. Proc Natl Acad Sci USA 84:7119–7123
Sipe DM, Jesurum A, Murphy RF (1991) Absence of Na+,K(+)-ATPase regulation of endosomal acidification in K562 erythroleukemia cells. Analysis via inhibition of transferrin recycling by low temperatures. J Biol Chem 266:3469–3474
Sluijs P van der, Hull M, Webster P, Male P, Goud B, Mellman I (1992) The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70:729–740
Sonawane ND, Verkman AS (2003) Determinants of [Cl-] in recycling and late endosomes and Golgi complex measured using fluorescent ligands. J Cell Biol 160:1129–1138
Tabuchi M, Yoshimori T, Yamaguchi K, Yoshida T, Kishi F (2000) Human NRAMP2/DMT1, which mediates iron transport across endosomal membranes, is localized to late endosomes and lysosomes in HEp-2 cells. J Biol Chem 275:22220–22228
Tooze J, Hollinshead M (1991) Tubular early endosomal networks in AtT20 and other cells. J Cell Biol 115:635–653
Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG (1996) Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135:913–924
Weert AW van, Dunn KW, Gueze HJ, Maxfield FR, Stoorvogel W (1995) Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol 130:821–834
Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR (1984) Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell 37:789–800
Zen K, Biwersi J, Periasamy N, Verkman AS (1992) Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. J Cell Biol 119:99–110
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Austrian Science Fund P12967 and P17590 to R.F.
Rights and permissions
About this article
Cite this article
Baravalle, G., Schober, D., Huber, M. et al. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res 320, 99–113 (2005). https://doi.org/10.1007/s00441-004-1060-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1060-x