Abstract
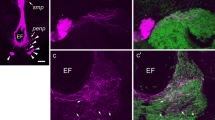
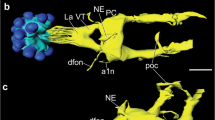
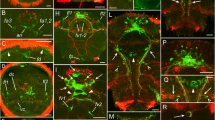
Material detectable with antisera to the pigment-dispersing hormone (PDH) is regarded as a component of the circadian clock residing in some insects in the optic lobe. This paper demonstrates that the position of the PDH-positive neurones and the course of their processes are similar in all representatives of the insect cohort Polyneoptera. A basic morphological pattern, which includes the proximal frontoventral (Pfv), distal posteriodorsal (Dpd) and posterioventral (Dpv) clusters of PDH-positive neurones, was found in the examined species of locusts, crickets, walking sticks, cockroaches, earwigs and termites. The Pfv cluster is located close to the accessory medulla and usually consists of a set of smaller and a set of larger perikarya. The Dpd and Dpv clusters occupy a dorsal and a ventral position, respectively, at the distal edge of the medulla. These clusters are lacking in stonefly and praying mantid species. The fan-like arrangement of PDH-positive fibres within the frontal medulla face (the locusts and the praying mantid have an additional, smaller fan on the posterior medulla face) is another characteristic feature of Polyneoptera. One (two in the locusts and the praying mantid) nerve bundle runs from the optic lobe to the lateral protocerebrum where it ramifies. One branch gives rise to a fibre network frontally encircling brain neuropile in the area of mushroom bodies. One thin fibre in the crickets and the earwig, and several thicker and anastomosing fibres in the other insects, connect the brain hemispheres. The arrangement of other PDH-positive structures specifies taxa within Polyneoptera. Specific features comprise the presence of PDH-positive perikarya in protocerebrum (walking stick and termite), deutocerebrum (cricket, walking stick, and one cockroach species), tritocerebrum (another cockroach species), and the suboesophageal ganglion (cricket, walking stick and termite). In the walking stick and the termite, PDH-positive fibres pass from the cephalic to the frontal ganglion and from there via the recurrent nerve to the corpora cardiaca where they make varicosities indicative of peptide release into the haemolymph.






Similar content being viewed by others
References
Chen B, Meinertzhagen IA, Shaw SR (1999) Circadian rhythms in light-evoked responses of the fly's compound eye, and the effects of neuromodulators 5-HT and the peptide PDF. J Comp Physiol [A] 185:393–404
Colwell CS, Page TL (1990) A circadian rhythm in neural activity can be recorded from the central nervous system of the cockroach. J Comp Physiol [A] 166:643–649
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP (1987) The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands—identification by an antiserum against a synthetic PDH. Cell Tissue Res 250:377–387
Frisch B, Fleissner G, Brandes C, Hall JC (1996) Staining in the brain of Pachymorpha sexguttata mediated by an antibody against a Drosophila clock-gene product: labelling of cells with possible importance for the beetle's circadian rhythms. Cell Tissue Res 286:411–429
Hagberg M (1986) Ultrastructure and central projections of extraocular photoreceptors in caddisflies (Insecta: Trichoptera). Cell Tissue Res 245:643–648
Hall JC (1998) Genetics of biological rhythms in Drosophila. Adv Genet 33:135–184
Helfrich-Förster C (1995) The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci U S A 92:612–616
Helfrich-Förster C (1997) Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol 380:335–354
Helfrich-Förster C, Homberg U (1993) Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol 337:177–190
Helfrich-Förster C, Stengl M, Homberg U (1998) Organization of the circadian system in insects. Chronobiol Int 15:567–594
Helfrich-Förster C, Täuber M, Park JH, Mühlig-Versen M, Schneuwly S, Hofbauer A (2000) Ectopic expression of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster. J Neurosci 20:3339–3353
Homberg U, Würden S, Dircksen H, Rao KR (1991a) Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res 266:343–357
Homberg U, David NT, Hildebrand JG (1991b) Peptide-immunocytochemistry of neurosecretory cells in the brain and retrocerebral complex of the sphinx moth Manduca sexta. J Comp Neurol 303:35–52
Jackson FR, Schroeder AJ, Roberts MA, McNeil GP, Kume K, Akten B (2001) Cellular and molecular mechanisms of circadian control in insects. J Insect Physiol 47:833–842
Meinertzhagen IA, Pyza E (1996) Daily rhythms in cells of the fly's optic lobe: taking time out from the circadian clock. Trends Neurosci 19:285–291
Nässel DR, Shiga S, Wikstrand EM, Rao KR (1991) Pigment-dispersing hormone-immunoreactive neurons and their relation to serotonergic neurons in the blowfly and cockroach visual system. Cell Tissue Res 266:511–523
Nässel DR, Shiga S, Mohrherr CJ, Rao KR (1993) Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. J Comp Neurol 331:183–198
Nishiitsutsuji-Uwo J, Pittendrigh CS (1968a) Central nervous system control of circadian rhythmicity in the cockroach. II. The pathway of light signals that entrain the rhythm. Z Vgl Physiol 58:1–13
Nishiitsutsuji-Uwo J, Pittendrigh CS (1968b) Central nervous system control of circadian rhythmicity in the cockroach. III. The optic lobes, locus of the driving oscillation? Z Vgl Physiol 58:14–46
Page TL (1981) Effects of localized low-temperature pulses on the cockroach circadian pacemaker. Am J Physiol 240:R144–R150
Page TL (1982) Transplantation of the cockroach circadian pacemaker. Science 216:73–75
Page TL (1983) Regeneration of the optic tracts and circadian pacemaker activity in the cockroach Leucophaea maderae. J Comp Physiol 152:231–240
Page TL, Caldarola PC, Pittendrigh CS (1977) Mutual entrainment of bilaterally distributed circadian pacemakers. Proc Natl Acad Sci U S A 74:1277–1281
Park JH, Helfrich-Förster C, Lee G, Liu L, Rosbash M, Hall JC (2000) Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A 97:3608–3613
Persson MGS, Eklund MB, Dircksen H, Muren JE, Nässel DR (2001) Pigment-dispersing factor in the locust abdominal ganglia may have roles as circulating neurohormone and central neuromodulator. J Neurobiol 48:19–41
Petri B, Stengl M (1997) Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. J Neurosci 17:4087–4093
Petri B, Stengl M, Würden S, Homberg U (1995) Immunocytochemical characterization of the accessory medulla in the cockroach Leucophaea maderae. Cell Tissue Res 282:3–19
Rao KR, Riehm JP (1988) Pigment-dispersing hormones: a novel family of neuropeptides from arthropods. Peptides 9:153–159
Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons both cause severe abnormalities of circadian behavioral rhythms in Drosophila. Cell 99:791–802
Sauman I, Reppert SM (1996) Circadian clock neurons in the silkmoth Antheraea pernyi: novel mechanisms of period protein regulation. Neuron 17:889–900
Shiga S, Rao KR, Nässel DR (1993) Pigment-dispersing hormone immunoreactive neurons in the blowfly nervous system. Acta Biol Hung 44:55–59
Sokolove PG (1975) Localization of the cockroach optic lobe circadian pace-maker with microlesions. Brain Res 87:13–21
Sokolove PG, Loher W (1975) Role of eyes, optic lobes and pars intercerebralis in locomotory and stridulatory circadian rhythms of Teleogryllus commodus. J Insect Physiol 21:785–799
Stengl M, Homberg U (1994) Pigment-dispersing hormone-immunoreactive neurons in the cockroach Leucophaea maderae share properties with circadian pacemaker neurons. J Comp Physiol [A] 175:203–213
Tomioka K, Chiba Y (1992) Characterization of optic lobe circadian pacemaker by in situ and in vitro recording of neuronal activity in the cricket Gryllus bimaculatus. J Comp Physiol [A] 171:1–7
Wheeler WC, Whiting M, Wheeler QD, Carpenter JM (2001) The phylogeny of extant hexapod orders. Cladistics 17:113–169
Wise S, Davis NT, Tyndale E, Noveral J, Folwell MG, Bedian V, Emery IF, Siwicki KK (2002) Neuroanatomical studies of period gene expression in the hawkmoth, Manduca sexta. J Comp Neurol 447:366–380
Závodská R, Sauman I, Sehnal F (2003) Distribution of PER protein, pigment-dispersing hormone, prothoracicotropic hormone, and eclosion hormone in the cephalic nervous system of insects. J Biol Rhythms (in press)
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was done in the framework of project 204/98/0043 of the Grant Agency of the Czech Republic and projects A5007205 and K5052113 of the Grant Agency of the Academy of Sciences
Rights and permissions
About this article
Cite this article
Sehadová, H., Sauman, I. & Sehnal, F. Immunocytochemical distribution of pigment-dispersing hormone in the cephalic ganglia of polyneopteran insects. Cell Tissue Res 312, 113–125 (2003). https://doi.org/10.1007/s00441-003-0705-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0705-5