Abstract
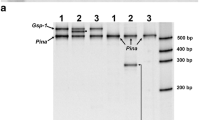
Wheat belongs to the most important crops domesticated in the Fertile Crescent. In this region, fortunately, locally adapted wheat landraces are still present in farmers’ fields. This material might be of immense value for future breeding programs. However, especially wheat germplasm adapted to the central part of the Fertile Crescent has been poorly characterized for allelic variation at key loci of agricultural importance. Grain hardness is an important trait influencing milling and baking quality of wheat. This trait is mainly determined by three tightly linked genes, namely, Puroindoline a (Pina), Puroindoline b (Pinb), and Grain softness protein-1 (Gsp-1), at the Hardness (Ha-D) locus on chromosome 5DS. To investigate genetic diversity and haplotype structure, we resequenced 96 diverse wheat lines at Pina-D1, Pinb-D1, Gsp-A1, Gsp-B1, and Gsp-D1. Three types of null alleles were identified using diagnostic primers: the first type was a multiple deletion of Pina-D1, Pinb-D1, and Gsp-D1 (Pina-D1k), the second was a Pina-D1 deletion (Pina-D1b); and the third type was a deletion of Gsp-D1, representing a novel null allele designated here as Gsp-D1k. Sequence analysis resulted in four allelic variants at Pinb-D1 and five at Gsp-A1, among them Gsp-A1-V was novel. Pina-D1, Gsp-B1 and Gsp-D1 sequences were monomorphic. Haplotype and phylogenetic analysis suggested that (1) bread wheat inherited its 5DS telomeric region probably from wild diploid Ae. tauschii subsp. tauschii found within an area from Transcaucasia to Caspian Iran; and that (2) the Ha-A and Ha-B homoeoloci were most closely related to sequences of wild tetraploid T. dicocco ides. This study provides a good overview of available genetic diversity at Pina-D1, Pinb-D1, and Gsp-1, which can be exploited to extend the range of grain texture traits in wheat.



Similar content being viewed by others
References
Akpinar BA, Lucas SJ, Vrana J, Budak H (2015) Sequencing chromosome 5D of Aegilops tauschii and comparison with its allopolyploid descendant bread wheat (Triticum aestivum). Plant Biotechnol J 13:740–752
Andeden EE, Yediay FE, Baloch FS, Shaaf S, Nachit M, Kilian B, Özkan H (2011) Distribution of vernalization and photoperiod genes (Vrn-A1, Vrn-B1, Vrn-D1, Vrn-B3, Ppd-D1) in Turkish bread wheat cultivars and landraces. Cereal Res Commun 39:352–364
Ayala M, Guzmán C, Alvarez J, Peña R (2013) Characterization of genetic diversity of puroindoline genes in Mexican wheat landraces. Euphytica 190:53–63
Bhave M, Morris CF (2008) Molecular genetics of puroindolines and related genes: allelic diversity in wheat and other grasses. Plant Mol Biol 66:205–219
Brown AHD, Weir BS (1983) Measuring genetic variability in plant populations. In: Tanksley SD, Orton TJ (eds) Isozymes in plant genetics and breeding, Part A. Elsevier, Amsterdam, pp 219–239
Bryant D, Moulton V (2004) Neighbor-Net: an agglomerative method for the construction of phylogenetic networks. Mol Biol Evol 21:255–265
Cane K, Spackman M, Eagles H (2004) Puroindoline genes and their effects on grain quality traits in southern Australian wheat cultivars. Austral J Agric Res 55:89–95
Chang C, Zhang H, Xu J, Li W, Liu G, You M, Li B (2006) Identification of allelic variations of puroindoline genes controlling grain hardness in wheat using a modified denaturing PAGE. Euphytica 152:225–234
Chantret N, Salse J, Sabot F et al (2005) Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). Plant Cell 17:1033–1045
Chao S, Dubcovsky J, Dvorak J, Luo MC, Baenziger SP, Matnyazov R, Clark DR, Talbert LE, Anderson JA, Dreisigacker S (2010) Population-and genome-specific patterns of linkage disequilibrium and SNP variation in spring and winter wheat (Triticum aestivum L.). BMC Genom 11:727
Charles M, Tang H, Belcram H, Paterson A, Gornicki P, Chalhoub B (2009) Sixty million years in evolution of soft grain trait in grasses: emergence of the softness locus in the common ancestor of Pooideae and Ehrhartoideae, after their divergence from Panicoideae. Mol Biol Evol 26:1651–1661
Chen F, He Z, Xia XC, Xia LQ, Zhang XY, Lillemo M, Morris CF (2006) Molecular and biochemical characterization of puroindoline a and b alleles in Chinese landraces and historical cultivars. Theor Appl Genet 112:400–409
Chen F, He Z, Chen D, Zhang C, Zhang Y, Xia X (2007a) Influence of puroindoline alleles on milling performance and qualities of Chinese noodles, steamed bread and pan bread in spring wheats. J Cereal Sci 45:59–66
Chen F, Yu Y, Xia X, He Z (2007b) Prevalence of a novel puroindoline b allele in Yunnan endemic wheats (Triticum aestivum ssp. yunnanense King). Euphytica 156:39–46
Devos KM, Ma J, Pontaroli AC, Pratt LH, Bennetzen JL (2005) Analysis and mapping of randomly chosen bacterial artificial chromosome clones from hexaploid bread wheat. Proc Natl Acad Sci USA 102:19243–19248
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Dvorák J, McGuire PE (1981) Nonstructural chromosome differentiation among wheat cultivars, with special reference to differentiation of chromosomes in related species. Genetics 97:391–414
Fu YX, Li WH (1993) Statistical tests of neutrality of mutations. Genetics 133:693–709
Gautier MF, Aleman ME, Guirao A, Marion D, Joudrier P (1994) Triticum aestivum puroindolines, two basic cysteine-rich seed proteins: cDNA sequence analysis and developmental gene expression. Plant Mol Biol 25:43–57
Gautier MF, Cosson P, Guirao A, Alary R, Joudrier P (2000) Puroindoline genes are highly conserved in diploid ancestor wheat and related species but absent in tetraploid Triticum species. Plant Sci 153:81–91
Giroux MJ, Morris CF (1997) A glycine to serine change in puroindoline b is associated with wheat grain hardness and low levels of starch-surface friabilin. Theor Appl Genet 95:857–864
Giroux MJ, Morris CF (1998) Wheat grain hardness results from highly conserved mutations in the friabilin components puroindoline-a and -b. Proc Natl Acad Sci USA 95:6262–6266
Giroux MJ, Talbert L, Habernicht DK, Lanning S, Hemphill A, Martin JM (2000) Association of puroindoline sequence type and grain hardness in hard red spring wheat. Crop Sci 30:370–374
Glaszmann JC, Kilian B, Upadhyaya HD, Varshney RK (2010) Accessing genetic diversity for crop improvement. Curr Opin Plant Biol 13(2):167–173
Greenwell P, Schofield JD (1986) A starch granule protein associated with endosperm softness in wheat. Cereal Chem 63:379–380
Guzmán C, Caballero L, Martín MA, Alvarez JB (2012) Molecular characterization and diversity of the Pina and Pinb genes in cultivated and wild diploid wheat. Mol Breed 30:69–78
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hartl DL, Clark AG (1997) Principles of population genetics, 3rd edn. Sinauer, Sunderland
Haudry A, Cenci A, Ravel C, Bataillon T, Brunel D, Poncet C, Hochu I, Poirier S, Santoni S, Glemin S (2007) Grinding up wheat: a massive loss of nucleotide diversity since domestication. Mol Biol Evol 24:1506–1517
Hudson RR, Kaplan NL (1985) Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics 111:147–164
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267
Ikeda TM, Ohnishi N, Nagamine T, Oda S, Hisatomi T, Yano H (2005) Identification of new puroindoline genotypes and their relationship to flour texture among wheat cultivars. J Cereal Sci 41:1–6
International Wheat Genome Sequencing Consortium (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345:1251788
Jia J, Zhao S, Kong X, Li Y, Zhao G, He W et al (2013) Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496:91–95
Jordan KW, Wang S, Lun Y et al (2015) A haplotype map of allohexaploid wheat reveals distinct patterns of selection on homoeologous genomes. Genome Biol 16:1–18
Kihara H (1944) Discovery of the DD-analyser, one of the ancestors of Triticum vulgare. Agric Hortic 19:13–14
Kilian B, Graner A (2012) NGS technologies for analyzing germplasm diversity in genebanks. Brief Funct Genomics 11:38–50
Kilian B, Özkan H, Deusch O, Effgen S, Brandolini A, Kohl J, Martin W, Salamini F (2007a) Independent wheat B and G genome origins in outcrossing Aegilops progenitor haplotypes. Mol Biol Evol 24:217–227
Kilian B, Özkan H, Walther A, Kohl J, Dagan T, Salamini F, Martin W (2007b) Molecular diversity at 18 loci in 321 wild and 92 domesticate lines reveal no reduction of nucleotide diversity during Triticum monococcum (einkorn) domestication: implications for the origin of agriculture. Mol Biol Evol 24:2657–2668
Kilian B, Özkan H, Pozzi C, Salamini F (2009) Domestication of the Triticeae in the Fertile Crescent. In: Feuillet C, Muehlbauer GJ (eds) Genetics and genomics of the Triticeae, plant genetics and genomics: crops and models 7. Springer, Berlin, pp 81–119
Law CN, Young CF, Brown JWS, Snape JW, Worland JW (1978) The study of grain protein control in wheat using whole chromosome substitution lines. Seed protein improvement by nuclear techniques. International Atomic Energy Agency, Vienna, pp 483–502
Li W, Huang L, Gill BS (2008) Recurrent deletions of puroindoline genes at the grain Hardness locus in four independent lineages of polyploid wheat. Plant Physiol 146:200–212
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Lillemo M, Morris CF (2000) A leucine to proline mutation in puroindoline b is frequently present in hard wheats from Northern Europe. Theor Appl Genet 100:1100–1107
Lillemo M, Chen F, Xia X, William M, Peña RJ, Trethowan R, He Z (2006) Puroindoline grain hardness alleles in CIMMYT bread wheat germplasm. J Cereal Sci 44:86–92
Lopes MS, El-Basyoni I, Baenziger PS, Singh S, Royo C, Ozbek K, Aktas H, Ozer E, Ozdemir F, Manickavelu A, Ban T, Vikram P (2015) Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J Exp Bot. doi:10.1093/jxb/erv122
Lucas S, Akpinar BA, Simkova H, Kubalakova M, El Dole J, Budak H (2014) Next-generation sequencing of flow-sorted wheat chromosome 5D reveals lineage-specific translocations and widespread gene duplications. BMC Genom 15:1080
Lyford C, Kidd W, Duarte PR, Deyoe C (2005) Prediction of flour extraction in a hard red winter wheat using the single kernel characterization. J Food Qual 28:279–288
Ma D, Zhang Y, Xia X, Morris CF, He ZH (2009) Milling and Chinese raw white noodle qualities of common wheat near-isogenic lines differing in puroindoline b alleles. J Cereal Sci 50:126–130
Marcussen T, Sandve SR, Heier L, Spannagl M, Pfeifer M, Jakobsen KS, Wulff BBH, Steuernagel B, Mayer KFX, Olsen OA, Sequencing IWG (2014) Ancient hybridizations among the ancestral genomes of bread wheat. Science 345:1250092–1250102
Martynov SP, Dobrotvorskaya TV, Hon I, Faberova I (2006) Wheat-Pedigree and identified alleles of genes-on line. N.I. Vavilov Institute of Plant Industry, St. Petersburg and Crop Research Institute, Praha. http://genbank.vurv.cz/wheat/pedigree/default.htm. Accessed 7 Mar 2014
Massa AN, Morris CF (2006) Molecular evolution of the puroindoline-a, puroindoline-b, and grain softness protein-1 genes in the tribe Triticeae. J Mol Evol 63:526–536
Massa AN, Morris CF, Gill BS (2004) Sequence diversity of puroindoline-a, puroindoline-b, and the grain softness protein genes in Aegilops tauschii Coss. Crop Sci 44:1808–1816
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers WJ, Morris CF, Appels R, Xia XC (2013) Catalogue of gene symbols for wheat. In: 12th international wheat genetic symposium, 8–13 September 2013, Yokohama
Morris CF (2002) Puroindolines: the molecular genetic basis of wheat grain hardness. Plant Mol Biol 48:633–647
Morris CF, Rose SP (1996) Wheat. In: Henry RJ, Kettlewell PS (eds) Cereal grain quality. Chapman Hall, London, pp 3–54
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Özkan H, Willcox G, Graner A, Salamini F, Kilian B (2011) Geographic distribution and domestication of wild emmer wheat (Triticum dicoccoides). Genet Resour Crop Evol 58:11–53
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539
Pflüger LA, D’Ovidio R, Margiotta B, Peña R, Mujeeb-Kazi A, Lafiandra D (2001) Characterisation of high- and low-molecular weight glutenin subunits associated to the D genome of Aegilops tauschii in a collection of synthetic hexaploid wheats. Theor Appl Genet 103:1293–1301
Pickering P, Bhave M (2007) Comprehensive analysis of Australian hard wheat cultivars shows limited puroindoline allele diversity. Plant Sci 172:371–379
Qamar ZU, Bansal UK, Dong CM, Alfred RL, Bhave M, Bariana HS (2014) Detection of puroindoline (Pina-D1 and Pinb-D1) allelic variation in wheat landraces. J Cereal Sci 60:610–616
Rahman S, Jolly CJ, Skerritt JH, Wallosheck A (1994) Cloning of a wheat 15 kDa grain softness protein (GSP). GSP is a mixture of different puroindoline-like polypeptides. Eur J Biochem 223:917–925
Ram S, Boyko E, Giroux MJ, Gill BG (2002) Null mutation in puroindoline a is present in Indian wheats: puroindoline genes are located in the distal part of 5DS. J Plant Biochem Biotechnol 11:79–83
Simeone MC, Gedye KR, Mason-Gammer R, Gill BS, Morris CF (2006) Conserved regulatory elements identified from a comparative puroindoline gene sequence survey of Triticum and Aegilops diploid taxa. J Cereal Sci 44:21–33
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tanaka H, Morris CF, Haruna M, Tsujimoto H (2008) Prevalence of puroindoline alleles in wheat varieties from eastern Asia including the discovery of a new SNP in puroindoline b. Plant Genet Resour 6:142–152
Tranquilli G, Lijavetzky D, Muzzi G, Dubcovsky J (1999) Genetic and physical characterization of grain texture-related loci in diploid wheat. Mol Gen Genet 262:846–850
Tranquilli G, Heaton J, Chicaiza O, Dubcovsky J (2002) Substitutions and deletions of genes related to grain hardness and their effect on grain texture. Crop Sci 42:1812–1817
Turner M, Mukai Y, Leroy P, Charef B, Appels R, Rahman S (1999) The Ha locus of wheat: identification of a polymorphic region for tracing grain hardness in crosses. Genome 42:1242–1250
Wang J, Luo MC, Chen Z, You FM, Wei Y, Zheng Y, Dvorak J (2013) Aegilops tauschii single nucleotide polymorphisms shed light on the origins of wheat D-genome genetic diversity and pinpoint the geographic origin of hexaploid wheat. New Phytol 198:925–937
Watterson GA (1975) On the number of segregating sites in genetical models without recombination. Theor Popul Biol 7:256–276
Yediay FE, Andeden EE, Baloch FS, Börner A, Kilian B, Özkan H (2011) The allelic state at the major semi-dwarfing genes in a panel of Turkish bread wheat cultivars and landraces. Plant Genet Resour 9:423–429
Yücel C, Baloch FS, Özkan H (2009) Genetic analysis of some physical properties of bread wheat grain (Triticum aestivum L. em. Thell.). Turk J Agric For 33:525–535
Zohary D, Hopf M, Weiss E (2012) Domestication of plants in the old world: the origin and spread of domesticated plants in Southwest Asia, Europe, and the Mediterranean Basin, 4th edn. Oxford University Press, Oxford
Acknowledgments
We thank Frank Blattner for discussions, Birgit Kränzlin for excellent technical support and the sequencing services at IPK Gatersleben and at Max-Planck Institute for Plant Breeding Research Cologne for their excellent job. The authors would like to acknowledge the financial support given by TÜBİTAK (The Scientific and Technological Research Council of Turkey, TOVAG-107O207) and by IPK Gatersleben.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Hohmann.
B. Kilian and H. Özkan shared last authors.
Data accessibility
Sequence data have been deposited in GenBank Data library under accession numbers KC585009–KC585020 (Table 2).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shaaf, S., Sharma, R., Baloch, F.S. et al. The grain Hardness locus characterized in a diverse wheat panel (Triticum aestivum L.) adapted to the central part of the Fertile Crescent: genetic diversity, haplotype structure, and phylogeny. Mol Genet Genomics 291, 1259–1275 (2016). https://doi.org/10.1007/s00438-016-1180-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-016-1180-5