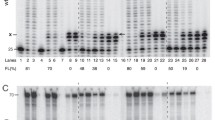
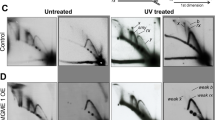
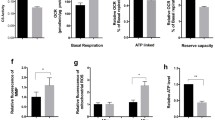
Abstract
How the cellular amount of mitochondrial DNA (mtDNA) is regulated under normal conditions and in the presence of genotoxic stress is less understood. We demonstrate that the inefficient mtDNA replication process of mutant yeast cells lacking the PIF1 DNA helicase is partly rescued in the absence of the DNA helicase RRM3. The rescue effect is likely due to the increase in the deoxynucleoside triphosphates (dNTPs) pool caused by the lack of RRM3. In contrast, the Pif1p-dependent mtDNA breakage in the presence and absence of genotoxic stress is not suppressed if RRM3 is lacking suggesting that this phenotype is likely independent of the dNTP pool. Pif1 protein (Pif1p) was found to stimulate the incorporation of dNTPs into newly synthesised mtDNA of gradient-purified mitochondria. We propose that Pif1p that acts likely as a DNA helicase in mitochondria affects mtDNA replication directly. Possible roles of Pif1p include the resolution of secondary DNA and/or DNA/RNA structures, the temporarily displacement of tightly bound mtDNA-binding proteins, or the stabilization of the mitochondrial replication complex during mtDNA replication.





Similar content being viewed by others
References
Bandy B, Davison AJ (1990) Mitochondrial mutations may increase oxidative stress: implications for carcinogenesis and aging? Free Radic Biol Med 8:523–539
Bessler JB, Torres JZ et al (2001) The Pif1p subfamily of helicases: region specific DNA helicases. Trends Cell Biol 11:60–65
Boule JB, Zakian VA (2007) The yeast Pif1p DNA helicase preferentially unwinds RNA DNA substrates. Nucleic Acids Res 35:5809–5818
Boule JB, Vega LR et al (2005) The yeast Pif1p helicase removes telomerase from telomeric DNA. Nature 438:57–61
Brewer BJ, Fangman WL (1987) The localization of replication origins on ARS plasmids in S. cerevisiae. Cell 51:463–471
Brewer BJ, Sena EP et al (1988) Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Cancer Cells 6:229–234
Budd ME, Reis CC et al (2006) Evidence suggesting that Pif1 helicase functions in DNA replication with the DNA2 helicase/nuclease and DNA polymerase delta. Mol Cell Biol 26:2490–2500
Chen XJ, Wang X et al (2005) Aconitase couples metabolic regulation to mitochondrial DNA maintenance. Science 307:714–717
Cheng X, Dunaway S et al (2007) The role of Pif1p, a DNA helicase in Saccharomyces cerevisiae, in maintaining mitochondrial DNA. Mitochondrion 7:211–222
Copeland WC (2008) Inherited mitochondrial diseases of DNA replication. Annu Rev Med 59:131–146
Cortopassi GA, Arnheim N (1990) Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res 18:6927–6933
Doudican NA, Song B et al (2005) Oxidative DNA damage causes mitochondrial genomic instability in Saccharomyces cerevisiae. Mol Cell Biol 25:5196–5204
Duchniewicz M, Germaniuk A et al (1999) Dual role of the mitochondrial chaperone Mdj1p in inheritance of mitochondrial DNA in yeast. Mol Cell Biol 19:8201–8210
Elpeleg O, Mandel H et al (2002) Depletion of the other genome-mitochondrial DNA depletion syndromes in humans. J Mol Med 80:389–396
Enriquez JA, Ramos J et al (1994) Highly efficient DNA synthesis in isolated mitochondria from rat liver. Nucleic Acids Res 22:1861–1865
Falkenberg M, Larsson NG et al (2007) DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem 76:679–699
Ferguson LR, von Borstel RC (1992) Induction of the cytoplasmic ‘petite’ mutation by chemical and physical agents in Saccharomyces cerevisiae. Mutat Res 265:103–148
Foury F, Dyck EV (1985) A PIF-dependent recombinogenic signal in the mitochondrial DNA of yeast. EMBO J 4:3525–3530
Foury F, Kolodynski J (1983) pif mutation blocks recombination between mitochondrial rho+ and rho- genomes having tandemly arrayed repeat units in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 80:5345–5349
Foury F, Lahaye A (1987) Cloning and sequencing of the PIF gene involved in repair and recombination of yeast mitochondrial DNA. EMBO J 6:1441–1449
Foury F, Roganti T et al (1998) The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett 440:325–331
Futami K, Shimamoto A et al (2007) Mitochondrial and nuclear localization of human Pif1 helicase. Biol Pharm Bull 30:1685–1692
Hall RM, Nagley P et al (1976) Biogenesis of mitochondria XLII. Genetic analysis of the control of cellular mitochondrial DNA levels in Saccharomyces cerevisiae. Mol Gen Genet 145:169–175
Huberman JA, Spotila LD et al (1987) The in vivo replication origin of the yeast 2 mm plasmid. Cell 51:473–481
Ivessa AS, Zhou J-Q et al (2000) The Saccharomyces Pif1p DNA helicase and the highly related Rrm3p have opposite effects on replication fork progression in ribosomal DNA. Cell 100:479–489
Ivessa AS, Zhou J-Q et al (2002) Saccharomyces Rrm3p, a 5′ to 3′ DNA helicase that promotes replication fork progression through telomeric and sub-telomeric DNA. Genes Dev 16:1383–1396
Ivessa AS, Lenzmeier BA et al (2003) The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein–DNA complexes. Mol Cell 12:1525–1536
Kalifa L, Sia EA (2007) Analysis of Rev1p and Pol zeta in mitochondrial mutagenesis suggests an alternative pathway of damage tolerance. DNA Repair (Amst) 6:1732–1739
Korhonen JA, Pham XH et al (2004) Reconstitution of a minimal mtDNA replisome in vitro. EMBO J 23:2423–2429
Krishnan KJ, Reeve AK et al (2008) What causes mitochondrial DNA deletions in human cells? Nat Genet 40:275–279
Kujoth GC, Hiona A et al (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309:481–484
Kujoth GC, Bradshaw PC et al (2007) The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet 3:e24
Lahaye A, Stahl H et al (1991) PIF1: a DNA helicase in yeast mitochondria. EMBO J 10:997–1007
Lahaye A, Leterme S et al (1993) PIF1 DNA helicase from Saccharomyces cerevisiae. Biochemical characterization of the enzyme. J Biol Chem 268:26155–26161
Lai JS, Herr W (1992) Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci U S A 89:6958–6962
Larsen NB, Rasmussen M et al (2005) Nuclear and mitochondrial DNA repair: similar pathways? Mitochondrion 5:89–108
Lecrenier N, Foury F (1995) Overexpression of the RNR1 gene rescues Saccharomyces cerevisiae mutants in the mitochondrial DNA polymerase-encoding MIP1 gene. Mol Gen Genet 249:1–7
Lecrenier N, Foury F (2000) New features of mitochondrial DNA replication system in yeast and man. Gene 246:37–48
Longtine M, McKenzie AI et al (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961
MacAlpine DM, Perlman PS et al (1998) The high mobility group protein Abf2p influences the level of yeast mitochondrial DNA recombination intermediates in vivo. Proc Natl Acad Sci USA 95:6739–6743
Mandavilli BS, Santos JH et al (2002) Mitochondrial DNA repair and aging. Mutat Res 509:127–151
Mateyak MK, Zakian VA (2006) Human PIF helicase is cell cycle regulated and associates with telomerase. Cell Cycle 5:2796–2804
McKenzie D, Bua E et al (2002) Mitochondrial DNA deletion mutations: a causal role in sarcopenia. Eur J Biochem 269:2010–2015
Mookerjee SA, Lyon HD et al (2005) Analysis of the functional domains of the mismatch repair homologue Msh1p and its role in mitochondrial genome maintenance. Curr Genet 47:84–99
Moraes CT, Shanske S et al (1991) mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet 48:492–501
Murakami H, Pain D et al (1988) 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol 107:2051–2057
Myung K, Chen C et al (2001) Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411:1073–1076
Nagley P, Linnane AW (1972) Biogenesis of mitochondria XXI. Studies on the nature of the mitochondrial genome in yeast: the degenerative effects of ethidium bromide on mitochondrial genetic information in a respiratory competent strain. J Mol Biol 66:181–193
Noguchi E, Noguchi C et al (2003) Swi1 prevents replication fork collapse and controls checkpoint kinase Cds1. Mol Cell Biol 23:7861–7874
O’Rourke TW, Doudican NA et al (2002) Mitochondrial dysfunction due to oxidative mitochondrial DNA damage is reduced through cooperative actions of diverse proteins. Mol Cell Biol 22:4086–4093
O’Rourke TW, Doudican NA et al (2005) Differential involvement of the related DNA helicases Pif1p and Rrm3p in mtDNA point mutagenesis and stability. Gene 354:86–92
Perlman PS, Mahler HR (1971) Molecular consequences of ethidium bromide mutagenesis. Nat New Biol 231:12–16
Prokisch H, Scharfe C et al (2004) Integrative analysis of the mitochondrial proteome in yeast. PLoS Biol 2:795–804
Rose MD, Winston F et al (1990) Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schiestl RH, Gietz RD (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16:339–346
Schinkel AH, Tabak HF (1989) Mitochondrial RNA polymerase: dual role in transcription and replication. Trends Genet 5:149–154
Schulz VP, Zakian VA (1994) The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 76:145–155
Sickmann A, Reinders J et al (2003) The proteome of Saccharomyces cerevisiae mitochondria. Proc Natl Acad Sci USA 100:13207–13212
Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19–27
Snow BE, Mateyak M et al (2007) Murine Pif1 interacts with telomerase and is dispensable for telomere function in vivo. Mol Cell Biol 27:1017–1026
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Stevnsner T, Thorslund T et al (2002) Mitochondrial repair of 8-oxoguanine and changes with aging. Exp Gerontol 37:1189–1196
Stith CM, Sterling J et al (2008) Flexibility of eukaryotic okazaki fragment maturation through regulated strand displacement synthesis. J Biol Chem 283:34129–34140
Taylor RW, Turnbull DM (2005) Mitochondrial DNA mutations in human disease. Nat Rev Genet 6:389–402
Taylor SD, Zhang H et al (2005) The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae. Mol Biol Cell 16:3010–3018
Trifunovic A, Wredenberg A et al (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429:417–423
Van Dyck E, Foury F et al (1992) A single-stranded DNA binding protein required for mitochondrial DNA replication in S cerevisiae is homologous to E. coli SSB. EMBO J 11:3421–3430
Wagner M, Price G et al (2006) The absence of Top3 reveals an interaction between the Sgs1 and Pif1 DNA helicases in Saccharomyces cerevisiae. Genetics 174:555–573
Wellinger RE, Prado F et al (2006) Replication fork progression is impaired by transcription in hyperrecombinant yeast cells lacking a functional THO complex. Mol Cell Biol 26:3327–3334
Xia T, Korge P et al (2004) Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect 112:1347–1358
Zhang DH, Zhou B et al (2006) The human Pif1 helicase, a potential Escherichia coli RecD homologue, inhibits telomerase activity. Nucleic Acids Res 34:1393–1404
Zhao X, Muller EG et al (1998) A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell 2:329–340
Zhao X, Chabes A et al (2001) The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J 20:3544–3553
Zhou J-Q, Monson EM et al (2000) The Pif1p helicase, a catalytic inhibitor of telomerase lengthening of yeast telomeres. Science 289:771–774
Acknowledgments
We thank Drs. Ronald Butow, Donna Gordon, Debkumar Pain, and Carolyn Suzuki for providing reagents. We are especially grateful to Drs. Donna Gordon, Chee-Gun Lee, Debkumar Pain, and Carolyn Suzuki for discussions and advice in cell biological aspects of mitochondria. This work is supported by a startup grant from the Department of Cell Biology and Molecular Medicine of the University of Medicine and Dentistry NJ, by a grant from the Foundation of the University of Medicine and Dentistry, NJ, and by a grant from the New Jersey Commission on Cancer Research (05-1975-CCR-EO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Aguilera.
X. Cheng, Y. Qin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cheng, X., Qin, Y. & Ivessa, A.S. Loss of mitochondrial DNA under genotoxic stress conditions in the absence of the yeast DNA helicase Pif1p occurs independently of the DNA helicase Rrm3p. Mol Genet Genomics 281, 635–645 (2009). https://doi.org/10.1007/s00438-009-0438-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-009-0438-6