Abstract
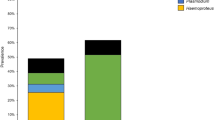
Sedentary bird species are suitable model hosts for identifying potential vectors of avian blood parasites. We studied haemosporidian infections in the Tengmalm’s Owl (Aegolius funereus) in the Ore Mountains of the Czech Republic using molecular detection methods. Sex of owl nestlings was scored using molecular sexing based on fragment analysis of PCR-amplified CHD1 introns. Observed infection prevalences in nestlings and adult owls were 51 and 86 %, respectively. Five parasite lineages were detected. Most of the infections comprised the Leucocytozoon AEFUN02 and STOCC06 lineages that probably refer to distinct Leucocytozoon species. Other lineages were detected only sporadically. Mixed infections were found in 49 % of samples. The main factor affecting the probability of infection was host age. No effect of individual sex on infection probability was evidenced. The youngest infected nestling was 12 days old. High parasite prevalence in the Tengmalm’s Owl nestlings suggests that insect vectors must enter nest boxes to transmit parasites before fledging. Hence, we placed sticky insect traps into modified nest boxes, collected potential insect vectors, and examined them for the presence of haemosporidian parasites using molecular detection. We trapped 201 insects which were determined as biting midges from the Culicoides genus and two black fly species, Simulium (Nevermannia) vernum and Simulium (Eusimulium) angustipes. Six haemosporidian lineages were detected in the potential insect vectors, among which the Leucocytozoon lineage BT2 was common to the Tengmalm’s Owl and the trapped insects. However, we have not detected the most frequently encountered Tengmalm’s Owl Leucocytozoon lineages AEFUN02 and STOCC06 in insects.


Similar content being viewed by others
References
Appleby BM, Anwar MA, Petty SJ (1999) Short-term and long-term effects of food supply on parasite burdens in Tawny Owls, Strix aluco. Funct Ecol 13:315–321
Bantock TM, Prys-Jones RP, Lee PLM (2008) New and improved molecular sexing methods for museum. Mol Ecol Resour 8:519–528
Beadell JS, Covas R, Gebhard C, Ishtiaq F, Melo M, Schmidt BK, Perkins SL, Graves GR, Fleischer RC (2009) Host associations and evolutionary relationships of avian blood parasites from West Africa. Int J Parasitol 39:257–266
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358
Bishop MA, Bennett GF (1992) Host-parasite catalogue of the avian haematozoa, supplement 1, and bibliography of the avian blood-inhabiting haematozoa, supplement 2. Meml Univ Nfld Occas Pap Biol 15:1–244
Chakarov N, Linke B, Boerner M, Goesmann A, Krüger O, Hoffman JI (2015) Apparent vector-mediated parent-to-offspring transmission in an avian malaria-like parasite. Mol Ecol 2015:1355–1363
Chvála M (1980) Fauna ČSSR, vol 22. Academia, Prague
Dimitrov D, Zehtindjiev P, Bensch S (2010) Genetic diversity of avian blood parasites in SE Europe: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Bulgaria. Acta Parasitol 55:201–209
Garamszegi LZ (2010) The sensitivity of microscopy and PCR-based detection methods affecting estimates of prevalence of blood parasites in birds. J Parasitol 96:1197–1203
Griffith R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Gutiérrez RJ (1989) Hematozoa from the spotted owl. J Wildl Dis 25:614–618
Halekoh U, Hojsgaard S, Yan J (2006) The R package geepack for generalized estimating equations. J Stat Softw 15:1–11
Hasselquist D, Östman Ö, Waldenström J, Bensch S (2007) Temporal patterns of occurrence and transmission of the blood parasite Haemoproteus payevskyi in the great reed warbler Acrocephalus arundinaceus. J Ornithol 148:401–409
Hayward GD, Hayward PH (1992) Tengmalm’s Owl. In: Poole A (ed) The birds of North America. American Ornithologists’ Union, Washington, D.C
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802
Ilmonen P, Hakkarainen H, Koivunen V, Korpimäki E, Mullie A, Shutler D (1999) Parental effort and blood parasitism in Tengmalm’s owl: effects of natural and experimental variation in food abundance. OIKOS 86:79–86
Ishak HD, Dumbacher JP, Anderson NL, Keane JJ, Valkiūnas G, Haig SM, Tell LA, Sehgal RNM (2008) Blood parasites in owls with conservation implications for the Spotted Owl (Strix occidentalis). PLoS ONE 3, e2304
Jenkins T, Owens GI (2011) Biogeography of avian blood parasites (Leucocytozoon spp.) in two resident hosts across Europe: phylogeographic structuring or the abundance–occupancy relationship? Mol Ecol 20:3910–20
Korpimäki E, Hakkarainen H (2012) The Tengmalm’s Owl: ecology, behaviour and conservation of a forest-dwelling predator. Cambridge University Press, Cambridge
Korpimäki E, Hakkarainen H, Bennett GF (1993) Blood parasites and reproductive success of Tengmalm’s owls: detrimental effects on females but not on males? Funct Ecol 7:420–426
Krone O, Waldenström J, Valkiūnas G, Lessow O, Müller K, Iezhova TA, Fickel J, Bensch S (2008) Haemosporidian blood parasites in European birds of prey and owls. J Parasitol 94:709–715
Martínez-de la Puente J, Martínez J, Rivero-de Aguilar J, Herrero J, Merino S (2011) On the specificity of avian blood parasites: revealing specific and generalist relationships between haemosporidians and biting midges. Mol Ecol 20:3275–3287
Martínez-de la Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E, García-Fraile S, Belda EJ (2010) The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett 6:663–665
Mendes L, Pardal S, Morais J, Antunes S, Ramos JA, Pérez-Tris J, Piersma T (2013) Hidden haemosporidian infections in ruffs (Philomachus pugnax) staging in Northwest Europe en route from Africa to Arctic Europe. Parasitol Res 112:2037–2043
Monahan WB, Hijmans RJ (2007) Distributional dynamics of invasion and hybridization by Strix spp. in western North America. Ornithol Monogr 63:55–66
Ortego J, Cordero PJ (2009) PCR-based detection and genotyping of haematozoa (Protozoa) parasitizing eagle owls, Bubo bubo. Parasitol Res 104:467–470
Ortego J, Cordero PJ (2010) Factors associated with the geographic distribution of leucocytozoa parasitizing nestling eagle owls (Bubo bubo): a local spatial-scale analysis. Conserv Genet 11:1479–1487
Outlow DC, Ricklefs RE (2009) On the phylogenetic relationships of haemosporidian parasites from raptorial birds (Falconiformes and Strigiformes). J Parasitol 95:1171–1176
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Remple JD (2004) Intracellular hematozoa of raptors: a review and update. J Avian Med Surg 18:75–88
Sonerud (1985) Nest hole shift in Tengmalm’s owl (Aegolius funereus) as defence against nest predation involving long-term memory in the predator. J Anim Ecol 54:179–192
Synek P, Albrecht T, Vinkler M, Schnitzer J, Votýpka J, Munclinger P (2013a) Haemosporidian parasites of a European passerine wintering in South Asia: diversity, mixed infections and effect on host condition. Parasitol Res 112:1667–1677
Synek P, Munclinger P, Albrecht T, Votypka J (2013b) Avian haemosporidians in haematophagous insects in the Czech Republic. Parasitol Res 112:839–845
Tomás G, Merino S, Martínez-de la Puente J, Moreno J, Morales J, Lobato E (2008) A simple trapping method to estimate abundances of blood-sucking flying insects in avian nests. Anim Behav 75:723–729
Tomás G, Merino S, Martínez-de la Puente J, Moreno J, Morales J, Lobato E, Rivero-de Aguilar J, Del Cerro S (2012) Interacting effects of aromatic plants and female age on nest-dwelling ectoparasites and blood-sucking flies in avian nests. Behav Processes 90:246–253
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiūnas G (2011) Haemosporidian vector research: marriage of molecular and microscopical approaches is essential. Mol Ecol 20:3084–3086
van Rooyen J, Lalubin F, Glaizot O, Christe P (2013) Altitudinal variation in haemosporidian parasite distribution in great tit populations. Parasit Vectors 6:139
Votýpka J, Synek P, Svobodová M (2009) Endophagy of biting midges attacking cavity-nesting birds. Med Vet Entomol 23:277–280
Acknowledgments
This study was supported by the Czech Science Foundation (GA ČR), grant no. P506/10/0716, and the Internal Grant Agency of Czech University of Life Sciences Prague, grant no. SVV 260 208/2015. We acknowledge the help of our colleagues Václav Tomášek, Marek Kouba, Petra Menclová, and Jan Hanel in the field and thank Zdena Csiebreiová for her technical assistance in the laboratory.
Ethical standards
This study was performed under a certificate of competency according to §17 of Act No. 246/1992 Coll., on the protection of animals against cruelty (registration number CZU 945/05), and complies with the current law of the Czech Republic.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Synek, P., Popelková, A., Koubínová, D. et al. Haemosporidian infections in the Tengmalm’s Owl (Aegolius funereus) and potential insect vectors of their transmission. Parasitol Res 115, 291–298 (2016). https://doi.org/10.1007/s00436-015-4745-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4745-z