Abstract
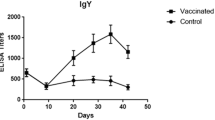
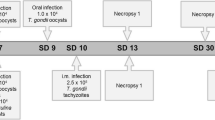
Toxoplasma gondii is a parasite which can be transmitted to humans via the consumption of contaminated meat products derived from different animal species, e.g., poultry. In Europe, the consumption rate of poultry meat is high and may pose a risk for humans. However, little is known about the prevalence and immune response against T. gondii in these animals. Based on these circumstances, we experimentally infected 18 turkeys and 16 chickens with the parasite. Turkeys were infected either with tachyzoites on different routes or with various amounts of oocysts. In contrast, chickens were only infected with different doses of oocysts. The immunoglobulin (Ig) Y humoral immune responses of these animals were investigated in a lineblot assay against the recombinant T. gondii antigens rGRA1, rGRA6, rGRA9, rSAG1, and rSUB1. By using the recombinant antigens rGRA6, rGRA9, and rSUB1 in the lineblot assay, we found a correlation between the humoral immune response and the parasite stage in turkeys. Thereby, an infection with oocysts induced a stronger, permanent long-lasting antibody response compared to tachyzoite-infected animals. Only a minor relation between the oocyst infection dose and the manifestation of the immune response in chickens was found 7 days post infection (dpi) by using rGRA1 and rGRA9. However, an inconstant detection of antigen-specific IgY antibodies in the lineblot assay seems not to be a sufficient method for the identification of a Toxoplasma infection in chickens. In contrast, the detection of anti-rGRA6, anti-rGRA9, and anti-rSUB1 IgY antibodies showed potential for the identification of an infection in turkeys.



Similar content being viewed by others
References
Aubert D, Maine GT, Villena I, Hunt JC, Howard L, Sheu M, Brojanac S, Chovan LE, Nowlan SF, Pinon JM (2000) Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol 38(3):1144–1150
Bartova E, Sedlak K, Literak I (2009) Serologic survey for toxoplasmosis in domestic birds from the Czech Republic. Avian Pathol 38(4):317–320
Bouer A, Werther K, Machado RZ, Nakaghi AC, Epiphanio S, Catao-Dias JL (2010) Detection of anti-Toxoplasma gondii antibodies in experimentally and naturally infected non-human primates by Indirect Fluorescence Assay (IFA) and indirect ELISA. Rev Bras Parasitol Vet 19(1):26–31
Conde de Felipe MM, Molina JM, Rodriguez-Ponce E, Ruiz A, Gonzalez JF (2007) IGM and IGG response to 29–35-kDa Toxoplasma gondii protein fractions in experimentally infected goats. J Parasitol 93(3):701–703
Davison F, Kaspers B, Schat LA (2008) Avian immunology. Academic Press, London
Dubey JP (2010) Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health 57(1):60–73
Dubey JP, Camargo ME, Ruff MD, Wilkins GC, Shen SK, Kwok OC, Thulliez P (1993a) Experimental toxoplasmosis in turkeys. J Parasitol 79(6):949–952
Dubey JP, Ruff MD, Camargo ME, Shen SK, Wilkins GL, Kwok OC, Thulliez P (1993b) Serologic and parasitologic responses of domestic chickens after oral inoculation with Toxoplasma gondii oocysts. Am J Vet Res 54(10):1668–1672
Dubey JP, Edelhofer R, Marcet P, Vianna MC, Kwok OC, Lehmann T (2005) Genetic and biologic characteristics of Toxoplasma gondii infections in free-range chickens from Austria. Vet Parasitol 133(4):299–306
El-Massry A, Mahdy OA, El-Ghaysh A, Dubey JP (2000) Prevalence of Toxoplasma gondii antibodies in sera of turkeys, chickens, and ducks from Egypt. J Parasitol 86(3):627–628
Ferrandiz J, Mercier C, Wallon M, Picot S, Cesbron-Delauw MF, Peyron F (2004) Limited value of assays using detection of immunoglobulin G antibodies to the two recombinant dense granule antigens, GRA1 and GRA6 Nt of Toxoplasma gondii, for distinguishing between acute and chronic infections in pregnant women. Clin Diagn Lab Immunol 11(6):1016–1021
Golkar M, Azadmanesh K, Khalili G, Khoshkholgh-Sima B, Babaie J, Mercier C, Brenier-Pinchart MP, Fricker-Hidalgo H, Pelloux H, Cesbron-Delauw MF (2008) Serodiagnosis of recently acquired Toxoplasma gondii infection in pregnant women using enzyme-linked immunosorbent assays with a recombinant dense granule GRA6 protein. Diagn Microbiol Infect Dis 61(1):31–39
Gross U, Roos T, Appoldt D, Heesemann J (1992) Improved serological diagnosis of Toxoplasma gondii infection by detection of immunoglobulin A (IgA) and IgM antibodies against P30 by using the immunoblot technique. J Clin Microbiol 30(6):1436–1441
Gross U, Keksel O, Darde ML (1997) Value of detecting immunoglobulin E antibodies for the serological diagnosis of Toxoplasma gondii infection. Clin Diagn Lab Immunol 4(3):247–251
Harfoush M, Tahoon Ael N (2010) Seroprevalence of Toxoplasma gondii antibodies in domestic ducks, free-range chickens, turkeys and rabbits in Kafr El-Sheikh Governorate Egypt. J Egypt Soc Parasitol 40(2):295–302
Hruzik A, Asif AR, Gross U (2011) Identification of Toxoplasma gondii SUB1 antigen as a marker for acute infection by use of an innovative evaluation method. J Clin Microbiol 49(7):2419–2425
Koethe M, Pott S, Ludewig M, Bangoura B, Zoller B, Daugschies A, Tenter AM, Spekker K, Bittame A, Mercier C, Fehlhaber K, Straubinger RK (2011) Prevalence of specific IgG-antibodies against Toxoplasma gondii in domestic turkeys determined by kinetic ELISA based on recombinant GRA7 and GRA8. Vet Parasitol 180(3–4):179–190
Kotresha D, Noordin R (2010) Recombinant proteins in the diagnosis of toxoplasmosis. APMIS 118(8):529–542
Magor KE (2011) Immunoglobulin genetics and antibody responses to influenza in ducks. Dev Comp Immunol 35(9):1008–1016
Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD (2001) Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 167(8):4574–4584
Pietkiewicz H, Hiszczynska-Sawicka E, Kur J, Petersen E, Nielsen HV, Stankiewicz M, Andrzejewska I, Myjak P (2004) Usefulness of Toxoplasma gondii-specific recombinant antigens in serodiagnosis of human toxoplasmosis. J Clin Microbiol 42(4):1779–1781
Pinon JM, Toubas D, Marx C, Mougeot G, Bonnin A, Bonhomme A, Villaume M, Foudrinier F, Lepan H (1990) Detection of specific immunoglobulin E in patients with toxoplasmosis. J Clin Microbiol 28(8):1739–1743
Purzel J, Schmitt R, Viertlboeck BC, Gobel TW (2009) Chicken IgY binds its receptor at the CH3/CH4 interface similarly as the human IgA: Fc alpha RI interaction. J Immunol 183(7):4554–4559
Warr GW, Magor KE, Higgins DA (1995) IgY: clues to the origins of modern antibodies. Immunol Today 16(8):392–398
Yan C, Yue CL, Yuan ZG, Lin RQ, He Y, Yin CC, Xu MJ, Song HQ, Zhu XQ (2010) Molecular and serological diagnosis of Toxoplasma gondii infection in experimentally infected chickens. Vet Parasitol 173(3–4):179–183
Zoller B, Koethe M, Ludewig M, Pott S, Daugschies A, Straubinger RK, Fehlhaber K, Bangoura B (2013) Tissue tropism of Toxoplasma gondii in turkeys (Meleagris gallopavo) after parenteral infection. Parasitol Res 112(5):1841–1847
Acknowledgment
This work was supported by grants from the Federal Ministry of Education and Research (BMBF) within the TOXONET consortium (grant numbers 01 KI0766 and 01 KI1002B to U.G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hotop, A., Buschtöns, S., Bangoura, B. et al. Humoral immune responses in chickens and turkeys after infection with Toxoplasma gondii by using recombinant antigens. Parasitol Res 113, 1473–1480 (2014). https://doi.org/10.1007/s00436-014-3788-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3788-x