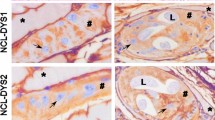
Abstract
The study evaluates the role of apoptosis-inducing factor (AIF) in the process of striated muscle cell transformation after occupation by Trichinella spiralis. Its relationship with other apoptosis-related factors [apoptotic protease-activating factor 1, Bcl-2 associated protein X (BAX), Bcl-2, caspase 3, survivin, poly (ADP-ribose) polymerase-1 (PARP-1), and endothelial and inducible (iNOS) nitric oxide synthase] was evaluated by immunohistochemistry. In the context of low BAX and caspase 3 expression and strong distribution of AIF in the sarcoplasm and nucleus at the very early stage of infection, we suppose that AIF-mediated signaling is involved in the apoptosis activation in the area of Trichinella occupation. In the time course of nurse cell formation, survivin and caspase 3 migrated into the enlarged nuclei with strong PARP-1 expression. In the end of encapsulation of Trichinella, expression of all proapoptotic factors ceased and only survivin in the nuclei and Bcl-2 positivity in the cytoplasm persisted in the formed nurse cell. The expression of sarcoplasmic iNOS was absent during the process of muscle cell de-differentiation and reappeared within the nurse cell. It seems that upregulation and downregulation of factors of apoptosis in the skeletal muscle cell represents an adaptive mechanism providing a comfortable niche for the parasite.


Similar content being viewed by others
References
Boonmars T, Wu Z, Nagano I, Takahashi Y (2005) Expression of apoptosis-related factors in muscles infected with Trichinella spiralis. Parasitol Res 97:13–20
Braga M, Sinha Hikim AP, Datta S, Ferrini MG, Brown D, Kovacheva EL, Gonzalez-Cadavid NF, Sinha-Hikim I (2008) Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis 13:822–832
Bruschi F, Murrel KD (2000) Tropical infectious diseases: principles, pathogens and practice. Churchill-Livingstone, Philadelphia
Butcher BA, Gagliardo LF, ManWarren T, Appleton JA (2000) Larvae-induced plasma membrane wounds and glycoprotein deposition are insufficient for Trichinella spiralis invasion of epithelial cells. Mol Biochem Parasitol 107:207–218
Candé C, Vahsen N, Garrido C, Kroemer G (2004) Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ 11:591–595
Despommier DD (1998) How does Trichinella spiralis make itself at home? Parasitol Today 14:318–323
Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA (1997) Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death. Involvement serine but not cysteine proteases. Am J Pathol 151:1205–1213
Dupont-Versteegden EE (2006) Apoptosis in skeletal muscle and its relevance to atrophy. World J Gastroenterol 12:7463–7466
Fukuda S, Pelus LM (2006) Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 5:1087–1098
Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG (1993) Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res 53:3976–3985
Khan K, Araki K, Wang D, Li G, Li X, Zhang J, Xu W, Hoover RK, Lauter S, O'Malley B Jr, Lapidus RG, Li D (2010) Head and neck cancer radiosensitization by the novel poly(ADP-ribose) polymerase inhibitor GPI-15427. Head Neck 32:381–391
Kołodziej-Sobocińska M, Dziemian E, Machnicka-Rowińska B (2006) Inhibition of nitric oxide production by aminoguanidine influences the number of Trichinella spiralis parasites in infected "low responders" (C57BL/6) and "high responders" (BALB/c) mice. Parasitol Res 99:194–196
Li CKF, Ko RC (2001) Inflammatory response during the muscle phase of Trichinella spiralis and T. pseudospiralis infections. Parasitol Res 87:708–714
Matsuo A, Wu Z, Nagano I, Takahashi Y (2000) Five types of nuclei present in the capsule of Trichinella spiralis. Parasitology 121:203–210
Norberg E, Orrenius S, Zhivotovsky B (2010) Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF). Biochem Biophys Res Commun 396:96–100
Siu PM, Pistilli EE, Butler DC, Alway SE (2005) Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol 288:338–349
Sui PM, Alway SE (2005) Mitochondria-associated apoptotic signaling in denervated rat skeletal muscle. J Physiol 565(1):309–323
Van Loo G, Saelens X, Van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P (2002) The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ 9:1031–1042
Wu Z, Nagano I, Boonmars T, Takahashi Y (2005) Tumor necrosis factor receptor-mediated apoptosis in Trichinella spiralis-infected muscle cells. Parasitology 131:705–712
Wu Z, Sofronic-Milosavljevic Lj, Nagano I, Takahasi Y (2008) Trichinella spiralis: nurse cell formation with emphasis on analogy to muscle cell repair. Parasit Vectors. doi:10.1186/1756-3305-1-27
Yao C, Jasmer DP (1998) Nuclear antigens in Trichinella spiralis infected muscle cells; nuclear extraction, compartmentalization and complex formation. Mol Biochem Parasitol 92:207–218
Yu SW, Wang H, Dawson TM, Dawson VL (2003) Poly (ADP-ribose) polymerase-1 and apoptosis inducing factor in neurotoxicity. Neurobiol Dis 14:303–317
Zara S, Rapino M, Centurione L, Di Giacomo V, Petruccelli G, Cataldi A (2010) Inducible nitric oxide synthase-activated mitochondrial apoptotic pathway in hypoxic and aged rat hearts. Gerontology 56:544–552
Acknowledgements
The authors wish to acknowledge Mrs. Ivica Uhnava and Mrs. Emilia Klincova for their excellent technician assistance. This study was supported in part by the grant MZ SR No. 2006-UK-02 from the Ministry of Health of the Slovak Republic, the Operational program Development of Human Resources-2007–2013, funded by the European Social Fund and the Republic of Bulgaria and by the Framework Programme for Research and Technology Development, project: Building of Centre of Excellency for Sudden Cerebral Vascular Events, Comenius University Faculty of Medicine in Bratislava (ITMS: 26240120023), co-financed by the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babal, P., Milcheva, R., Petkova, S. et al. Apoptosis as the adaptation mechanism in survival of Trichinella spiralis in the host. Parasitol Res 109, 997–1002 (2011). https://doi.org/10.1007/s00436-011-2343-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2343-2