Abstract
A complete cDNA encoding a 21.1-kDa tegumental protein (CsTP21.1) was recognized from Clonorchis sinensis adult full-length cDNA plasmid library by bioinformatics analysis. Recombinant CsTP21.1 was highly expressed in Escherichia coli, purified by affinity chromatography, and identified by Western blotting. Immunohistochemistry demonstrated that CsTP21.1 is localized in the tegument of the adult worm. The rCsTP21.1-specific IgG1, IgG2, and IgG4 subclasses could be detected in the sera of clonorchiasis patients by ELISA, but their sensitivity was much lower than that of total IgG. The sensitivity and specificity of IgG in 66 serum samples of clonorchiasis patients were 100% and 95.5%, and the sensitivity was independent of worm loads; the cross-reaction rates in 86, 24, and 31 serum samples from patients infected with Fasciola hepatica, Schistosoma japonicum, and nematode were 98.8%, 83.3%, 93.3%, respectively, whereas no cross-reactions with Toxoplasma gondii and sparganum. This study demonstrated that CsTP21.1 is a trematode–nematode pan-specific antigen that is valuable in the development of a universal immunodiagnostic kit for human trematode and nematode infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clonorchis sinensis is a food-borne zoonotic parasite and mainly distributes in Korea, China, Vietnam, and the eastern part of Russia. It is estimated that about 35 million people are infected globally, of which approximately 15 million are in China, especially in southern and northeastern provinces (Lun et al. 2005; Rim 2005). In recent years, the morbidity is rapidly rising due to the prevalence of eating raw freshwater fish which usually contain living metacercariae, rendering clonorchiasis an important public health problem (Fang et al. 2000). C. sinensis infection causes subclinical or clinical hepatic and biliary diseases, and heavy or long-term chronic infections often lead to cholelithiasis, pyogenic cholangitis, cholecystitis, hepatic fibrosis, hepatic neoplasm, and even cholangiocarcinoma (Liao et al. 2006; Lin et al. 1987).
Clonorchiasis diagnosis mainly depends on light microscopy examination of eggs in feces, which is not convenient and efficient in clinical laboratory inspection and epidemiological survey. Therefore, feasible immunodiagnostic method is urgently needed (Ambroise-Thomas and Goullier 1984). In recent years, a series of C. sinensis antigens, such as 7-kDa antigen (Kim et al. 1998; Zhao et al. 2004), cystatin (Kim et al. 2001), glutathione S-transferase (Hong et al. 2002), and cysteine proteinase (Nagano et al. 2004) have been identified and produced by genetic engineering for the development of diagnostic kit; however, for most of antigens, their sensitivity and specificity is not satisfactory.
Our laboratory had constructed a full-length cDNA plasmid library of C. sinensis adult, from which a large number of antigen genes including those encoding tegumental proteins were identified (Xu et al. 2004). The tegument is a special syncytium structure of platyhelminth, an interface between parasite and host, and a source of released antigens. The tegumental proteins are easily released to stimulate host immune response and are considered as candidates of diagnostic antigens (Sobhon et al. 1998; Van Hellemond et al. 2006; Qian and Hu 2008; Mulvenna et al. 2010). Tegumental proteins are the focus of platyhelminth studies in understanding host–parasite interactions as well as developing diagnostic kits and vaccines (Mohamed et al. 1998; Fitzsimmons et al. 2004; Cardoso et al. 2006, 2008; Lopes et al. 2009). From C. sinensis cDNA library, four tegumental proteins of C. sinensis were identified, of which CsTP20.8 (Zhou et al. 2007), CsTP31.8 (Huang et al. 2007), and CsTP22.3 (Zhou et al. 2008a, b) vaccine development or serodiagnosis potentials were evaluated. They are unstable antigens and easily degrade without enough sensitivity in serodiagnosis. This paper describes another C. sinensis tegumental protein, CsTP21.1, for its localization and immunological characteristics.
Materials and methods
Bioinformatics analysis
Among 1,775 C. sinensis adult unigenes, one (clone no. Cs0010c03) was predicted as a member of tegumental protein family using the basic local alignment search tool (BLAST) at the National Center for Biotechnology Information website (http://ncbi.nlm.nih.gov) and InterProScan program (http://www.ebi.ac.uk/Tools/InterProScan/). The physicochemical parameters of putative protein was predicted by the online program ProtParam (http://www.expasy.ch/tools/protparam.html), subcellular localization by TargetP (http://www.cbs.dtu.dk/services/Target), tertiary structure by SWISS-MODEL (http://swissmodel.expasy.org/), and antigenic determinants by IEDB Analysis Resource (http://tools.immuneepitope.org).
Cloning, expression, purification, and immunological identification
The coding sequence of CsTP21.1 was amplified by polymerase chain reaction (PCR) using 5′ primer (5′-CGGCATATGATGGAACCGTTTATGGATGC-3′) and 3′ primer (5′-ATTCTCGAGGGTTTTGATGATGTTTGGC-3′) with NdeI/XhoI restriction enzyme sites (underlined), respectively. Amplification went on 35 cycles under conditions of 94°C for 1 min, 53°C for 1 min, and 72°C for 1 min using Taq polymerase (Takara Shuzo Co., Kyoto, Japan). The PCR product was purified and cloned into the corresponding restriction sites of a prokaryotic expression vector pET28a(+) (Novagen) and the recombinant vector was confirmed by double-enzyme digestion and sequencing. The recombinant plasmid was transformed into Escherichia coli BL21(DE3) (Promega) and was induced to express protein by isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 1.0 mM for 5 h at 37°C. Then induced bacterial cells were then collected by centrifugation and broken by ultrasonication.
The rCsTP21.1 was expressed completely in inclusion bodies, which were collected and solubilized in 20 mM Tris–HCl buffer solution (pH 8.0) containing 8 M urea and 10% sodium dodecyl sulfate (SDS). Purification was performed in denatured condition using His Bind Purification kit (Novagen) according to the instruction of the manufacturer. The purified protein was renatured by dialysis through stepwise dilution of urea in 20 mM Tris–HCl and 5 mM EDTA buffer (pH 8.0). Protein samples were subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by Coomassie brilliant blue G-250.
Purified rCsTP21.1 (5 μg) was subjected to SDS-PAGE (12% gel) and then electrotransferred to polyvinylidene difluoride membrane (Qbiogene).The membranes were blocked with phosphate-buffered saline 0.05% Tween 20 (PBS-T, pH 7.4) containing 1% bovine serum albumin (BSA) overnight at 4°C and then incubated with clonorchiasis patient serum or health person serum at 1:200 dilution for 2 h at room temperature (RT). After washing five times with PBS-T, membranes were probed with horseradish peroxidase (HRP)-conjugated antirat immunoglobulin G (Fab specific, Boster, 1:2,000 dilution) for 1 h at RT. Both primary and secondary antibodies were diluted with 0.1% BSA in PBS-T. After washing again, membranes were developed with diaminobenzidine solution (Boster) as substrate.
Rat antirecombinant CsTP21.1 immune sera
Purified rCsTP21.1 (100 μg) was mixed with an equal volume of complete Freund’s adjuvant and then injected subcutaneously into the Sprague–Dawley rat and boosted twice with an equal amount of the antigen mixed with incomplete Freund’s adjuvant, at an interval of 2 weeks. Blood was collected to prepare antiserum at 1 week after the last injection.
Immunolocalization of CsTP21.1 at adult worm of C. sinensis
Sectioned worms in paraffin wax were deparaffinized and incubated in the rat anti-CsTP21.1 sera (1:100 dilution). Preimmune rat sera were employed to make a negative control. The sections were subsequently incubated in fluorescein isothiocyanate (FITC)-conjugated antirat IgG (1:50; Boster) and then observed under fluorescence microscope.
Collection of human serum
Sixty-six sera from clonorchiasis patients with different worm loads which is determined by a quantitative egg examination by Stoll’s method, according to eggs per gram (EPG) of stool, were judged as light (EPG <1,000), mild 1,000≤ EPG <10,000, and heavy (EPG ≥10,000). Another 66 control sera from healthy persons are judged by no eggs and no history of eating raw or undercooked freshwater fish in endemic areas. Sixteen Toxoplasma gondii-positive sera and six sparganum-positive sera were determined by enzyme-linked immunosorbent assay (ELISA) kits. All these sera samples were collected from the First Affiliated Hospital of Sun Yat-sen University. Eighty-six Fasciola hepatica-, 31 nematode-, and 24 Schistosoma japonicum-infected sera confirmed by egg examination were provided by the center of disease prevention and control of Gansu, Fujian, Jiangxi Province, respectively. All these sera donators gave informed consent.
Human antibody isotypes
From the 66 sera of clonorchiasis patients, 24 sera were randomly taken out to measure the levels of IgG and its subclasses by ELISA. In brief, microtiter plates (Corning Co.) were coated with 0.5 μg purified rCsTP21.1 antigen in 0.1 M carbonate–bicarbonate buffer (pH 9.6) per well and incubated at 4°C overnight and then blocked with 5% skim milk in PBS-T for 2 h at 37°C. One-hundred-microliter serum samples diluted 1:100 with PBS-T containing 0.1% BSA were added per well and incubated at 37°C for 2 h. One-hundred-microliter HRP-conjugated goat antihuman IgG (1:20,000, Dingguo, Beijing) was incubated for 0.5 h at 37°C and then developed color with substrate 3,3′,5,5′-tetramethylbenzidine. Its absorbance was measured at 450 nm after adding 2 M H2SO4 (50 μl per well) to stop the reaction. The IgG isotypes (including IgG1, IgG2, and IgG4) against rCsTP21.1 were measured as above at 1:50 dilution for sera and 1:2,000 (Southern Biotech Co.) dilution for secondary antibodies.
IgG ELISA sensitivity, specificity, and cross-reactivity
The rCsTP21.1-specific IgG level of 66 healthy human control sera, 66 clonorchiasis patient sera with light, mild, and heavy worm burden (22 for each group), and 86, 31, 24,16, and six serum samples from patients infected with F. hepatica, nematode, S. japonicum, T. gondii, and sparganum, respectively, were detected in ELISA according to the method described above. The absorbance value was calculated as a mean of the duplicate read for each sample. The cutoff point was determined by the mean optical density value plus three folds of standard deviation of the control group. The statistical software SPSS13.0 was used to analyze the data.
Result
Sequence analysis of CsTP21.1
BLASTx recognized the C. sinensis EST clone Cs0010c03 which encodes a putative tegumental protein with 39%, 31%, and 22% identity to the tegumental calcium-binding proteins from S. japonicum, F. hepatica, and C. brenneri, respectively. The complete coding sequence was 543 bp in length, coding 181 amino acids with the theoretical molecular weight of 21,145.9 Da, so it is named CsTP21.1. The sequence was submitted to GenBank (access no. HM020389). The InterProScan program found out two EF hand domains at the N terminus (amino acid Met1-Asn34 and amino acid Pro43-Asp68) and a dynein light-chain domain (Glu74-Ala172) in CsTP21.1. IEDB analysis resource predicted four strong linear B cell epitopes: aa46–52, aa71–82, aa110–118, and aa171–179 (Fig. 1). The ProtParam program predicted the theoretical pI of 4.63; the grand average of hydropathicity and the aliphatic index indicated that it is a hydrophobic protein. The TargetP predicted that the protein is present in cytoplasm. Swiss-Model modulated the 3D structure of the two EF hand domains. Each domain consists of two perpendicular α helixes (H) and a linked loop (L) which binds a calcium ion and is conjugated by a turn (Fig. 2).
Expression, purification, and identification of CsTP21.1
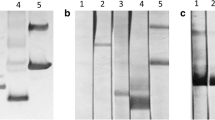
The recombinant plasmid pET-28a(+)-CsTP21.1 expressed a fusion protein with a 6× His tag at the N terminus in E. coli (BL21) under IPTG induction. The lysate of induced host bacteria SDS-PAGE showed an obvious additional band with the molecular weight (MW) close to theoretic MW (Fig. 3a). The rCsTP21.1 was only expressed in inclusion body in sediment of sonicated bacteria, not in supernatant. After denaturation in 8 mol/l urea and 10% SDS solution, the dialysis gradually decreased against urea solution; the recombinant protein was solely purified by Ni-IDA column affinity chromatography. The purified recombinant protein can be recognized by the clonorchiasis patient serum in Western blotting (Fig. 3b)
Expression, purification, and identification of recombinant CsTP21.1. a CsTP21.1 expression and purification demonstrated in SDS-PAGE. M protein marker, 1 before IPTG induction, 2 after IPTG induction, 3 purified recombinant protein. b rCsTP21.1 identified by Western blot. 1 healthy sera; 2 clonorchiasis patient sera
CsTP21.1 is a matrix protein in the tegument of C. sinensis adult worm
Fluorescence microscopy analysis using rat anti-rCsTP21.1 serum revealed strong fluorescence in the tegument of adult worm and only weak fluorescence appears in tegumental cells under the tegument (Fig. 4a), whereas rCsTP21.1 could not be detected in sections incubated with preimmune rat serum (Fig. 4b). This cytoplasmic antigen appears to be expressed in the matrix tegument of this helminth but absent in the internal tissues of the C. sinensis.
Immunolocalization of CsTP21.1 on C. sinensis tegument. (×400). Fluorescence microscopy images (left) and corresponding optical images (right) of tegument and underlying tissues of adult worm sections are shown. Polyclonal anti-CsTP21.1 immune rat sera and secondary antibody coupled to FITC demonstrated CsTP21.1 located in the tegument and tegumental cell of adult worm (a). Naïve rat serum used as negative control did not show fluorescence (b)
Human IgG isotype response to CsTP21.1
The level of total IgG in all 24 sera from clonorchiasis patients is high; the specific IgG1, IgG2, and IgG4 isotypes all exist but with low titers (Fig. 5). Although the IgG1 and IgG4 are the predominant IgG subclasses, their sensitivity is much lower than that of specific total IgG (data not shown). Therefore, the IgG ELISA will be more sensitive than IgG subclass ELISA.
Sensitivity, specificity, and cross-reactivity of IgG ELISA based on rCsTP21.1
The ELISA results of all samples detected were shown in a scatter plot. For 22 positive sera of each light, mild, and heavy C. sinensis infection group, there are no significant differences in IgG level, but in 66 sera of egg native control group, the IgG level is much lower than that of C. sinensis infection groups. According to the cutoff value of 0.135964, the positivity rate of egg-negative control group is 4.5%, with 95.5% specificity. The sensitivity for clonorchiasis is 100% (66/66). The positivity rates for F. hepatica, S. japonicum, and geohelminth (roundworm, hook worm, and whipworm) are 98.8% (85/86), 83.3% (30/36), and 93.3% (29/31), respectively. However, none of the 16 T. gondii-infected sera is positive, and only one of the six sparganum-infected sera is weakly positive (Fig. 6). These results demonstrated that there is no correlation between the worm burden and antibody level. The rCsTP21.1 seems to be a trematode and nematode pan-specific antigen with the total sensitivity of 95.9%.
rCsTP21.1-based IgG ELISA for egg-negative sera and parasite-infected sera. 1 Egg-negative group (n = 66), 2 C. sinensis light infection group (n = 22), 3 C. sinensis mild infection group (n = 22), 4 C. sinensis heavy infection group (n = 22), 5 F. hepatica infection group (n = 86), 6 S. japonicum infection group (n = 36), 7 nematode infection group (n = 31), 8 T. gondii infection group (n = 16), 9 sparganum infection group (n = 6)
Discussion
In the present study, we report a novel C. sinensis tegumental protein CsTP21.1 characterized by two calcium-binding EF hand domains and a dynein light-chain-like domain at N- and C-terminal fragment, respectively. The renatured purified rCsTP21.1 can be recognized by C. sinensis-infected sera, indicating it maintains the natural antigenicity. Immunohistochemistry demonstrated that CsTP21.1 is intensively expressed in the tegument and weakly expressed in tegumental cells, which is similar with other three CsTP proteins (Zhou et al. 2007, 2008a, b; Huang et al. 2007). This phenomenon suggests that this protein is produced in tegumental cells, delivered to tegument, and accumulated there. Although its exact function is unknown, CsTP21.1 is speculated to play a role in movement and transporting materials to tegument. Bioinformatics analysis revealed neither classic secretory signal peptide nor non-classic information and nor transmembrane region in CsTP21.1, suggesting that it is a cytoplasmic protein in the matrix of tegument syncytium and can shed from worm through tegument renewal to stimulate immune response.
In spite of relative small molecular weight, rich T cell epitopes, and linear B cell epitopes that endue it, good antigenicity was predicted in CsTP21.1. In human, IgG1 or IgG2 production in B cells is determined by Th2 cytokines (IL-4, IL-5, IL-13, etc.) and Th1 cytokines (IL-12, IFN-γ), respectively. Thus, the specific IgG1 and IgG2 against an antigen reflect its ability in evoking Th2 and Th1 response. Helminth antigens, as well as allergens, elicit the production of IgG4 and IgE in vivo and in vitro in experimental models (Lobos et al. 2003); similarly, exposure of naive (non-pre-switched) B cells in vitro to IL-4 (and IL-13) and IL-10 affect production of IgG4 or IgE and IgG1 or IgG3, respectively (Garraud and Nutman 1996). The titers of specific IgG1 and IgG4 to CsTP21.1 are higher than that of IgG2, suggesting that in vivo it evokes Th2 predominant response, under these Th2-prone immunological niche of chronic C. sinensis-infected patient, production of specific IgG2 suggested that CsTP21.1 can also evoke Th1 response in vivo, and this is verified by the high level of IgG2a in rats immunized CsTP21.1 without any adjuvant (data not shown). Therefore, CsTP21.1 of complete immunogenicity can simultaneously evoke Th1 and Th2 responses, which is consistent with the bioinformatics prediction of immunological characteristics.
Although CsTP21.1-specific IgG1, IgG2, and IgG4 are all present in the sera of clonorchiasis patients, the titers are relatively low, some subclasses are negative in some samples. Therefore, rCsTP21.1-based IgG ELISA was adopted in clonorchiasis immunodiagnosis. The high sensitivity (100%) and specificity (95.5%) are encouraging in clonorchiasis epidemic area. Moreover, OD value is so discriminatory between infection and control group that it can be easily judged by the naked eye (Fig. 6). Nevertheless, the most interesting is that the rCsTP21.1 is nearly of the same sensitivity in detecting other trematode (F. hepatica and S. japonicum) and nematode infection while it is nearly completely negative for T. gondii and sparganum infection. From these results, CsTP21.1 can be viewed as a trematode–nematode pan-specific antigen with the sensitivity of 97.7%. According to this view, the three positive sera in egg-negative control group probably had been infected with some other geohelminths or tissue-parasitizing helminthes which do not produce eggs in intestine.
Cross-reactions mean that there are common antigens. CsTP21.1 belongs to a tegumental protein family; however, members in the same or different species do not appear to have high homology. For example, the most homologous protein to CsTP21.1 in GenBank is a tegumental protein of S. japonicum only with the identity of 39%. Due to the low homology, the common cross-reaction is speculated to depend on common conformational epitopes between tegumental proteins, for they share the same H–L–H fashion of EF hand domain as well as similar spatial conformation. This H–L–H structure is similar in their α-helical coiled coil motif; in general, this structure does not exhibit a folding problem. The α-helical coiled coil motif bears a characteristic seven-amino-acid residue repeat (abcdefg)n with hydrophobic residues located in a and d positions and hydrophilic residues generally elsewhere; an important known characteristic of these domains is that, taken separately from the whole protein, they frequently and readily fold into the same stable oligomeric structure (Hodges 1996). The α-helical coiled coil fragments are frequently recognized by conformation-dependent antibodies and can elicit antibodies against structurally “native” epitopes. The antibodies against conformational epitopes can easily cross-react with homologs of similar secondary structure (Villard et al. 2007). Every EF hand domain is comprised of two conjugated α-helical coiled coil motifs, which can explain why antibodies to tegumental proteins of low homology can recognize each other. Those containing calcium-binding EF hand and dynein light-chain-type domains are usually membrane-associated tegumental proteins. Their similar secondary structure or tertiary structure contributes to the common cross-reactions; in fact, when rCsTP211.1 is denatured, the cross-reaction is greatly reduced (data not shown).
It is puzzling that there is no cross-reaction with sparganum, a cestode, which is more closely relative than nematodes with trematode in phylogeny evolution and owns similar tegument structure. Blastx searches the databases of cestodes using CsTP21.1; no similar gene sequence is found, suggesting that tegumental proteins may not exist in cestodes. Whether all cestodes are defective tegumental proteins needs further studies to confirm.
The tegument constitutes parasite–host interface (Xavier et al. 1998; Van Hellemond et al. 2006), and many tegumental proteins with EF hand domain were identified and localized in tegument of S. japonicum (Waine et al. 1994), S. mansoni (Smyth et al. 2003), and F. gigantica (Ruiz de Equino et al. 1999).Those antigens are usually considered as diagnosis candidate antigens. Three C. sinensis tegumental proteins, TP20.8, TP22.3, and TP31.8 are described in our previous studies, and due to their instability, neither of these three recombinant antigens has enough sensitivity and specificity in clonorchiasis diagnosis.
In summary, as a sensitive trematode and nematode pan-specific antigen, rCsTP21.1 is valuable in the development of a sensitive immunodiagnostic kit for human multiple helminths infection, and it is useful in primary screening of helminth infections in clinical laboratory diagnosis and field epidemiological survey.
References
Ambroise-Thomas P, Goullier A (1984) Parasitological examinations and immunodiagnostic advances in fluke infections. Arzneimittelforschung 34:1129–1132
Cardoso FC, Pacífico RN, Mortara RA, Oliveira SC (2006) Human antibody responses of patients living in endemic areas for schistosomiasis to the tegumental protein Sm29 identified through genomic studies. Clin Exp Immunol 144:382–391
Cardoso FC, Macedo GC, Gava E, Kitten GT, Mati VL, de Melo AL, Caliari MV, Almeida GT, Venancio TM, Verjovski-Almeida S, Oliveira SC (2008) Schistosoma mansoni tegument protein Sm29 is able to induce a Th1-type of immune response and protection against parasite infection. PLoS Negl Trop Dis 2:e308
Fang YY, Pan B, Shi XC, Chen ZZ, Lin RX, Huang SY, Zhang XC, Deng ZH, Zhang QM, Liu YY, He Q (2000) Comparative analysis of two surveys of distribution of human parasites in Guangdong province. Strait J Prevent Med 6:32–33 (in Chinese)
Fitzsimmons CM, Stewart TJ, Hoffmann KF, Grogan JL, Yazdanbakhsh M, Dunne DW (2004) Human IgE response to the Schistosoma haematobium 22.6 kDa antigen. Parasite Immunol 26:371–376
Garraud O, Nutman TB (1996) The role of cytokines in human B-cell differentiation into immunoglobulin secreting cells. Bull Inst Pasteur 94:285–309
Hodges RS (1996) De novo design of alpha-helical proteins: basic research to medical applications. Biochem Cell Biol 74:133–154
Hong SJ, Yun Kim T, Gan XX, Shen LY, Sukontason K, Sukontason K, Kang SY (2002) Clonorchis sinensis: glutathione S transferase as a serodiagnostic antigen for detecting IgG and IgE antibodies. Exp Parasitol 101:231–233
Huang Y, Zhou Z, Hu X, Wei Q, Xu J, Wu Z, Yu X (2007) A novel tegumental protein 31.8 kDa of Clonorchis sinensis: sequence analysis, expression, and immunolocalization. Parasitol Res 102:77–81
Kim, SI (1998) A Clonorchis sinensis-specific antigen that detects active human clonorchiasis. Korean J Parasitol 36:37–45
Kim TY, Kang SY, Park SH, Sukontason K, Sukontason K, Hong SJ (2001) Cystatin capture enzymelinked immunosorbent assay for serodiagnosis of human clonorchiasis and profile of captured antigenic protein of Clonorchis sinensis. Clin Diagn Lab Immunol 8:1076–1080
Liao WC, Wang HP, Chiu HM, Chang CY, Lin JT (2006) Multiple hepatic nodules: rare manifestation of clonorchiasis. Journal of Gastroenterology and Hepatology 21:1497–1500
Lin AC, Chapman SW, Turner HR, Wofford JD Jr (1987) Clonorchiasis: an update. South Med J 80:919–922
Lobos NTB, Hothersall JS, Moncada S (2003) Elevated IgE to recombinant Brugia malayi glutamyl transpeptidase in patients with bancroftian filariasis: association with tropical pulmonary eosinophilia or with putative immunity. Infect Immun 71:747–753
Lopes DO, Paiva LF, Martins MA, Cardoso FC, Rajão MA, Pinho JM, Caliari MV, Correa-Oliveira R, Mello SM, Leite LC, Oliveira SC (2009) Sm21.6 a novel EF-hand family protein member located on the surface of Schistosoma mansoni adult worm that failed to induce protection against challenge infection but reduced liver pathology. Vaccine 27:4127–4135
Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY (2005) Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis 5:31–41
Mohamed MM, Shalaby KA, LoVerde PT, Karim AM (1998) Characterization of Sm20.8, a member of a family of schistosome tegumental antigens. Mol Biochem Parasitol 96:15–25
Mulvenna J, Moertel L, Jones MK, Nawaratna S, Lovas EM, Gobert GN, Colgrave M, Jones A, Loukas A, McManus DP (2010) Exposed proteins of the Schistosoma japonicum tegument. Int J Parasitol 40(5):543–554
Nagano I, Pei F, Wu Z, Wu J, Cui H, Boonmars T, Takahashi Y (2004) Molecular expression of a cysteine proteinase of Clonorchis sinensis and its application to an enzyme-linked immunosorbent assay for immunodiagnosis of clonorchiasis. Clin Diagn Lab Immunol 11:411–416
Qian MB, Hu W (2008) The structure and function of schistosome tegument and related proteomic study. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 26(6):466–471 (in Chinese)
Rim HJ (2005) Clonorchiasis: an update. J Helminthol 79:269–281
Ruiz de Equino AD, Machin A, Casais R, Castro AM, Boqa JA, Martin-Alonso JM, Parra F (1999) Cloning and expression in Escherichia coli of a Fasciola hepatica gene encoding a calcium binding protein. Mol Biochem Parasitol 101:13–21
Smyth D, McManus DP, Smout MJ, Laha T, Zhang W, Loukas A (2003) Isolation of cDNAs encoding secreted and transmembrane proteins from Schistosoma mansoni by a signal sequence trap method. Infect Immun 71:2548–2554
Sobhon P, Anantavara S, Dangprasert T, Viyanant V, Krailas D, Upatham ES, Wanichanon C, Kusamran T (1998) Fasciola gigantica: studies of the tegument as a basis for the developments of immunodiagnosis and vaccine. Southeast Asian J Trop Med Public Health 29:387–400
Van Hellemond JJ, Retra K, Brouwers JFHM, Balkom BWM, Yazdanbakhsh M, Shoemaker CB, Tielens AGM (2006) Function of tegument of schistosomes: clues from the proteome and lipidome. Int J Parasitol 36:691–699
Villard V, Agak GW, Frank G, Jafarshad A, Servis C, Nébié I, Sirima SB, Felger I, Arevalo-Herrera M, Herrera S, Heitz F, Bäcker V, Druilhe P, Kajava AV, Corradin G (2007) Rapid identification of malaria vaccine candidates based on α-helical coiled coil protein motif. PLoS One 2:e645
Waine GJ, Becker MM, Scott JC, Kalinna BH, Yang W, McManus DP (1994) Purification of a recombinant Schistosoma japonicum antigen homologous to the 22-kDa membrane-associated antigen of S. mansoni, a putative vaccine candidate against schistosomiasis. Gene 142:259–263
Xavier EM, Lucena-Silva N, Werkhauser RP, Franco GR, Santos RA, Simpson AJ, Abath FG (1998) The tegument of Schistosoma mansoni: genes, antigens and the host–parasite relationship. Mem Inst Oswaldo Cruz 93:85–86
Xu J, Hu X, Kang Y, Wu Z, Chen S, Xie Y, Yu X (2004) Construction of full-length gene expression library of Clonorchis sinensis adults and establishment of the gene expression pattern. Chinese Journal of Zoonoses 2:383–393
Zhao QP, Moon SU, Lee HW, Na BK, Cho SY, Kong Y, Jiang MS, Li AH, Kim TS (2004) Evaluation of Clonorchis sinensis recombinant 7-kilodalton antigen for serodiagnosis of clonorchiasis. Clin Diagn Lab Immunol 11:814–817
Zhou Z, Hu X, Huang Y, Hu H, Ma C, Chen X, Hu F, Xu J, Lu F, Wu Z, Yu X (2007) Molecular cloning and identification of a novel Clonorchis sinensis gene encoding a tegumental protein. Parasitol Res 101:737–742
Zhou Z, Xia H, Hu X, Huang Y, Li Y, Li L, Ma C, Chen X, Hu F, Xu J, Lu F, Wu Z, Yu X (2008a) Oral administration of a Bacillus subtilis spore-based vaccine expressing Clonorchis sinensis tegumental protein 22.3 kDa confers protection against Clonorchis sinensis. Vaccine 26:1817–1825
Zhou Z, Xia H, Hu X, Huang Y, Ma C, Chen X, Hu F, Xu J, Lu F, Wu Z, Yu X (2008b) Immunogenicity of recombinant Bacillus subtilis spores expressing Clonorchis sinensis tegumental protein. Parasitol Res 102:293–297
Acknowledgements
This work is supported by the China National Great Basic Research Program (973 program, no. 2010CB530003) and China National Natural Science Foundation (no. 30771887).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Xu, H., Zhang, Z. et al. Cloning and expression of 21.1-kDa tegumental protein of Clonorchis sinensis and human antibody response to it as a trematode–nematode pan-specific serodiagnosis antigen. Parasitol Res 108, 161–168 (2011). https://doi.org/10.1007/s00436-010-2050-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2050-4