Abstract
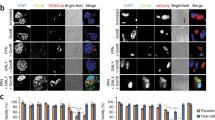
The susceptibility of Trypanosoma cruzi epimastigotes to lysis by normal or immune sera in a complement-dependent reaction has been reported. Mouse immune sera depleted complement-induced damage in epimastigotes characterized by morphological changes and death. The purpose of this work was to study the mechanism of death in epimastigotes exposed to decomplemented mouse immune serum. Epimastigotes were maintained in RPMI medium. Immune sera were prepared in mice by immunization with whole crude epimastigote extracts. Viable epimastigotes were incubated with decomplemented normal or immune sera at 37°C. By electron microscopy, agglutinated parasites showed characteristic patterns of membrane fusion between two or more parasites; this fusion also produced interdigitation of the subpellicular microtubules. Apoptosis was determined by flow cytometry using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and annexin V assays. Nuclear features were examined by 4′-,6-diamidino-2′-phenylindole diHCI cytochemistry that demonstrated apoptotic nuclear condensation. Caspase activity was also measured. TUNEL results showed that parasites incubated with decomplemented immune sera took up 26% of specific fluorescence as compared to 1.3% in parasites incubated with decomplemented normal sera. The Annexin-V-Fluos staining kit revealed that epimastigotes incubated with decomplemented immune sera exposed phosphatidylserine on the external leaflet of the plasma membrane. The incubation of parasites with immune sera showed caspase 3 activity. We conclude that specific antibodies are able to induce agglutination and apoptosis in epimastigotes, although the pathway is not elucidated.





Similar content being viewed by others
References
Ameisen JC, Idziorek TH, Billaut-Mulot O, Loyens M, Tissier JP, Potentier A, Ouaissi MA (1995) Apoptosis in a unicellular eukaryote (Trypanosoma cruzi): implications for the evolutionary origin and role of programmed cell death in the control of cell proliferation, differentiation and survival. Cell Death Differ 2:283–300
Barcinski MA, DosReis GA (1999) Apoptosis in parasites and parasite-induced apoptosis in the host immune system: a new approach for the parasitic diseases. Braz J Med Biol Res 32:395–401
Billaut-Mulot O, Fernandez-Gomez R, Loyens M, Ouaissi A (1996) Trypanosoma cruzi elongation factor 1-α: nuclear localization in parasites undergoing apoptosis. Gene 174:19–26
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Butler JE, Felbush TL, McGivern PL, Stwart N (1978) The enzyme-linked immunosorbent assay, ELISA. A measure of antibody concentration or affinity. Immunochem 15:131–136
Cohen JJ (1993) Apoptosis. Immunol Today 14:126–130
Cohen GM, Sun XM, Snowden RT, Dinsdale D, Skilleter DN (1992) Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J 286:331–334
Collins JA, Schandl CA, Young KK, Vesely J, Willingham MC (1997) Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem 45:923–934
Committee and Care and Use of Laboratory Animals (1996) Guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources. National Research Council, Washington, DC
Duvall E, Wyllie AH (1986) Death and the cell. Immunol Today 7:115–119
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Ann Review Biochem 6:383–424
Estaquier J, Idziorek T, De Bels F, Barré-Sinoussi F, Hurtrel B, Aubertin AM, Venet A, Mehtali M, Muchmore E, Michel P, Mouton Y, Girard M, Ameisen JC (1994) Programmed cell death and AIDS: the significance of T-cell apoptosis in pathogenic and non pathogenic primate lentiviral infections. Proc Natl Acad Sci U S A 91:9431–9435
Fadok VA, Bratton DL, Konowal A, Freed PW, Wescott JY, Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE-2 and PAF. J Clinical Inves 101:890–898
Fernández-Presas AM, Tay Z, Becker F, Merchant MT, Robert G, Willms K (2001) Ultrastructural damage of Trypanosoma cruzi epimastigotes exposed to decomplemented immune sera. Parasitol Res 87:619–625
Freire de Lima Celio G, Nascimento DO, Soares MB, Bozza PT, Castro-Faira-Neto HC, de Mello FG, DosReis GA, Lopes M (2000) Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403:199–203
Gavrieli Y, Sherman Y, Ben-Sasson SA (1988) Identification of a programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Gorczyca W, Gong J, Darzynkiewicz Z (1993) Detection of DNA strand breaks in individual apoptotic cells by in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res 53:1945–1951
Henriques-Pons A, Oliveira GM, Paiva MM, Correa AF, Batista MM, Bisaggio RC, Liu CC, Cotta-De-Almeida V, Coutinho CM, Persechini PM, Araújo-Jorge TC (2003) Evidence for a perforin-mediated mechanism controlling cardiac inflammation in Trypanosoma cruzi infection. Inter J Exp Path 83:67–79
Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D (1995) Human neutrophils lose their surface Fc gamma RIII and acquire annexin V binding sites during apoptosis in vitro. Blood 85:532–540
Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415–1420
Kosec G, Alvarez VE, Agüero F, Sánchez D, Dolinar M, Turk B, Turk V, Cazzulo JJ (2006) Metacaspases of Trypanosoma cruzi: possible candidates for programmed cell death mediators. Mol Bioch Parasitol 145:18–28
Kototani K, Kanbara H, Fukama T, Nakabayashi T (1979) Electron microscopic observations on lysis of Trypanosoma cruzi epimastigotes by normal rabbit serum. Biken J 22:109–115
Kowalczyk AGM, Horwacik I, Odrowaz Z, Kozbor D, Rokita H (2009) The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Lett 281:171–182
Lee N, Gannavaram S, Selvapandiyan A, Debrabant A (2007) Characterization of Metacaspases with trypsin-like activity and their putative role in programmed cell death in the protozoan parasite. Leishmania 6:1745–1757
Ming M, Ewen ME, Pereira MEA (1995) Trypanosome invasion of mammalian cells requires activation of the TGF-beta signaling pathway. Cell 8:287–296
Mpoke S, Wolfe J (1996) DNA digestion and chromatin condensation during nuclear death in Tetrahymena. Exp Cell Res 225:357–365
Muniz J, Boriello A (1945) Estudo sobre acao litica de diferentes soros sobre as formas de cultura e sanguícolas do Schizotrypanum cruzi. Revi Bras Biol 5:563–576
Nauta AJ, Daha MR, Tijsma O, van de Water B, Tedesco F, Roos A (2002) The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol 32:783–92
Nogueira N, Bianco C, Cohn Z (1975) Studies on the selective lysis and purification of Trypanosoma cruzi. J Exp Med 142:224–229
Ouaissi A (2003) Apoptosis-like death in trypanosomatids: search for putative pathways and genes involved. Kinetoplastid Biol Dis 2:1–5
Palomino SA, Aiello VD, Higuchi ML (2000) Systematic mapping of hearts from chronic patients: the association between the occurrence of histopathological lesions and Trypanosoma cruzi antigens. Ann Trop Med Parasitol 94:571–579
Powell MR, Kuhn RE (1980) Measurement of cytolytic antibody in experimental Chagas disease using a terminal radiolabeling procedure. J Parasitol 66:399–406
Rubio M (1956) Actividad litica de sueros normales sobre formas de cultivo e sanguícolas de Trypanosoma cruzi. Bol Chil Parasitol 11:62–69
Schaub GA (1994) Pathogenicity of trypanosomatids on insects. Parasitol Today 10:463–468
Shi Y (2002) Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 9:459–470
Silva JS, Twardzik DR, Reed SG (1991) Regulation of Trypanosoma cruzi infection in vitro and in vivo by transforming growth factor beta (TGF beta). J Exp Med 174:539–545
Steller H (1995) Mechanisms and genes of cellular suicide. Science 267:1445–1449
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Ucker DS, Obermiller PS, Eckhart W, Apgar JR, Berger NA, Meyers J (1992) Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol 12:3060–3069
Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EB, Dixit VM (2000) Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 6:961–967
Vaux DL (1993) Towards an understanding of the molecular mechanisms of physiological cell death. Pro Nat Acad Sci U S A 90:786–789
Vermes IC, Haanen H, Nakken S, Reutelingsperger C (1995) A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells. J Immunol Methods 17:39–51
Waghabi MC, Coutinho CM, Soeiro MN, Pereira MC, Feige JJ, Keramidas M, Cosson A, Minoprio P, Van-Leuven F, Araújo-Jorge TC (2002) Increased Trypanosoma cruzi invasion and heart fibrosis associated with high transforming growth factor beta levels in mice deficient in alpha (2)-macroglobulin. Infect Imm 70:5115–5123
Welburn SC (1999) Programmed cell death in procyclic form Trypanosoma brucei rhodesiense identification of differentially expressed genes during ConA induced death. Mem Inst Osw Cruz 94:229–234
Wyllie AH, Kerr JFR, Curie AR (1980) Cell death: the significance of apoptosis. Int Rev Cyt 68:251–306
Zakeri ZF, Quaglino D, Latham T, Lockshin RA (1993) Delayed internucleosomal DNA fragmentation in programmed cell death. FASEB J 7:470–478
Zangger H, Mottram JC, Fasel N (2002) Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ 10:1126–1139
Acknowledgments
We thank Dr. Horacio Merchant for the electronic micrographs and José Luis Ventura and Marco Gudiño for technical assistance.
This work was supported by grants IN-213208 DGAPA-UNAM and funding by the Departamento de Microbiología y Parasitología, Facultad de Medicina, Universidad Nacional Autónoma de México.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00436-010-1909-8
Rights and permissions
About this article
Cite this article
Fernández-Presas, A.M., Tato, P., Becker, I. et al. Specific antibodies induce apoptosis in Trypanosoma cruzi epimastigotes. Parasitol Res 106, 1327–1337 (2010). https://doi.org/10.1007/s00436-010-1803-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1803-4