Abstract
Blood samples were collected from 487 adult horses, including 83 pregnant mares, at a slaughterhouse located in Araguari, Minas Gerais State, Brazil. For each blood sample, the packed cell volume (PCV) was determined, and Giemsa-stained smears were microscopically examined for the presence of hemoparasites. The plasma was examined by the indirect fluorescent antibody test for detection of antibodies against Babesia caballi and Theileria equi. In addition, DNA was extracted and analyzed by a multiplex real-time polymerase chain reaction (PCR), specific for B. caballi and T. equi. Products of PCR were sequenced and compared with each other and with known sequences. The serological results showed a total prevalence of 91.0% for T. equi and 83.0% for B. caballi, while by PCR, prevalences of 59.7% for T. equi and 12.5% for B. caballi were observed. However, no correlations were seen between positivity (neither by serology nor by PCR) and PCV values. As expected, the microscopic examination of blood smears showed low sensitivity in detecting the infections when compared to the PCR. Only 35 out of 570 blood smears were positive, with parasitemias below 0.1%. No congenital transmission was detectable. As far as sequencing is concerned, no differences were seen among the isolates of each species nor among them and known sequences available. These results confirm, by molecular methods, the high prevalence rates of T. equi and B. caballi infections in carrier horses in Brazil. However, no diversity was observed among the isolates within the studied regions.
Similar content being viewed by others
Introduction
Equine babesioses are tick-borne diseases of domestic and wild equines caused by the exclusively intraerythrocytic protozoa Babesia caballi or by the intraerythrocytic and intralymphocytic protozoa Theileria equi (Friedhoff 1988; Uilenberg 2006). Anocentor nitens ticks are the vectors for B. caballi (Roby and Anthony 1963) and, in Brazil, it is known that Boophilus microplus can transmit T. equi (Guimaraes et al. 1998a, b; Heuchert et al. 1999; Battsetseg et al. 2002).
Clinical signs may vary from asymptomatic to acute cases with fever, anemia, edema, dyspnea, and death. Congenital transmission of T. equi can lead to abortion or neonatal death (Phipps and Otter 2004). Horses that recover from acute infections may remain reservoirs for ticks, which will transmit the infection to other susceptible horses. Several researchers postulate that B. caballi persists from 1 to 4 years in the host, whereas T. equi may remain as a lifelong infection (de Waal 1992).
Direct diagnosis can be made by microscopic examination of Giemsa-stained blood smears or by specific polymerase chain reactions (PCRs) (Böse et al. 1995). However, for epidemiological surveys, indirect methods, such as the indirect fluorescent antibody test (IFAT), are more appropriate to evaluate the status of a herd or an area. In tropical endemic countries, like Brazil, foals usually acquire the infections shortly after birth, and patent parasitemia are detected before 42 days of age (Ribeiro et al. 1995). Under these circumstances, symptomatic babesiosis is not expected to occur with high frequencies in adults, as they usually undergo subclinical or chronic infections.
Although Brazil is considered an endemic area, the epidemiological studies carried out to date are restricted to few areas and considered small numbers of samples for serological surveys. On the other hand, molecular characterization of Brazilian B. caballi and T. equi isolates is lacking.
Therefore, the aim of the present study was to determine, using direct and indirect methods, the prevalence of both infections in horses and to characterize molecularly Brazilian isolates of B. caballi and T. equi. In addition, congenital transmission was investigated.
Materials and methods
Location
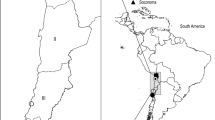
Collection of material was carried out in a horse slaughterhouse located in Araguari, Minas Gerais State, Brazil, where horse meat is processed for exportation. Horses were originally from the states of Minas Gerais, Goiás, Bahia, and São Paulo (Fig. 1). The permission for collection of samples was given by the official veterinarians inspecting the slaughterhouse. Blood samples from adult horses were collected before slaughtering, and fetus samples were collected after the slaughtering procedure to avoid any interference with the sanitary requirements of the Brazilian legislation and the international meat market.
Geographic location of the origin of horses within the states of Bahia (Ba), Goiás (Go), Minas Gerais (MG) and São Paulo (SP), including 3 municipalities in Bahia (Correntina, Caetité, and Itajú do Colônia), 9 in Goiás (São Domingos, Ipameri, Corumbaíba, Cachoeira Dourada, Quirinópolis, Santo Antônio da Barra, Indiara, and Arenópolis), 17 in Minas Gerais (Jaíba, Janaúba, São João da Ponte, Montes Claros, Patos de Minas, Uberlândia, São Sebastião do Paraíso, Guaxupé, Itajubá, Araguari, Ituiutaba, Conceição das Alagoas, Pedro Leopoldo, Caeté, Belo Horizonte, Conselheiro Lafaiete, and Três Pontas), and one in São Paulo (Luiziânia)
Collection and preparation of samples
Blood samples were collected from 487 adult horses, including 83 pregnant mares. Blood samples were also collected from the fetuses, which were measured to estimate the time of gestation, according to Ginther 1967.
Blood samples were collected in EDTA-tubes from the jugular vein of adult horses and from the heart of fetuses. For each blood sample, the packed cell volume (PCV) was determined, and Giemsa-stained blood smears were prepared for microscopic examination. DNA was extracted using a commercial Kit (Wizard Genomic DNA Purification, Promega, Madison, USA). Plasma samples were separated after centrifugation and were tested by IFAT for detection of antibodies against B. caballi and T. equi.
Molecular characterization
DNA was extracted from 300 μl of blood and eluated in 900 μl of buffer, following the manufacturer instructions. DNA concentrations were determined using the Nanodrop® ND-1000 spectrometer (Nanodrop Technologies, DE, USA). Detection of B. caballi and T. equi DNA was carried out using multiplex real-time PCR (MRT-PCR). Real-time PCR using targeting sequences of horse DNA was also run, as a reference, to guarantee the reliability of results. For detection of T. equi, the primers targeted a single-copy ema-1 gene (EMA 1 f/r), as referred by Ueti et al. 2003. For detection of B. caballi, primers were designed using the computer program “Beacon Designer 4.0” (BC 48 f/r). The two sets of primers were used in a modified MRT-PCR, in a way that they did not interfere with each other. The primer set for the horse DNA real-time PCR was also designed with the “Beacon Designer 4.0” program (HoCytBm-f/r). The primer sequences with their TaqMan® probes and the MRT-PCR conditions are summarized in Table 1.
For each batch of samples, a negative control (H2O) and positive controls (B. caballi and T. equi clones) were included.
For sequencing, some samples were randomly selected from different areas of the study, and a single target PCR was run using the primersets EMA1F (5′-TGCGCCATAGACGGAGAAGC-3′/R (5′-GCATCCATTGCCATTTCGAG-3′) and BC 48F (5′-GGCTCCCAGCGACTCTGTGG-3′)/R1 (5′TCTGCACTACCATAAGGGAGAAA-3′). Cycling was performed in a thermal cycler under the following conditions: 15 min/95°C; 15 s/94°C; 30 s/60°C; 45 s/72°C. Amplicons were visualized after electrophoresis of 5 μl of the PCR product on a 1.2% agarose gel stained with ethidium bromide. The PCR products were purified using commercial kits (E.Z.N.A.® Cycle-Pure Kit, Classic Line, peqLab, Biotechnologie GmbH, Erlangen, Germany; QIAquick Gel ExtractionKit, QUIAGEN, Hilden, Germany) and were sent directly for sequencing (Sequence Laboratories, Goettingen, Germany). Sequence alignments were performed using the “Sequence Manipulation Suite” http://www.bioformatics.org/sms/, BCM Multiple Alignment). Sequences of Brazilian B. caballi and T. equi isolates were also compared with sequences available in the GenBank http://www.ncbi.nlm.nih.gov).
Serology
Plasma samples were tested by IFAT, as described by Tenter and Friedhoff (1986) with modifications, at dilutions of 1:80, 1:160, 1:320, and 1:640. The antigen slides containing either T. equi or B. caballi had been previously produced at the Veterinary School, UFMG, Brazil, with infected blood collected from esplenectomized experimentally infected foals. A commercial antihorse IgG conjugate (Sigma, Germany) was used. For both parasites, the dilution of 1:80 was considered the cut-off point for seropositivity.
Statistical analysis
SPSS and Microsoft Excel® programs were used for descriptive statistics, and the qui-square test was used to analyze associations between positivity in blood smears or PCR and a low PCV, as well as the occurrence of concomitant infections, and positivity in the blood smear and early detection by the PCR.
Results
Detection of B. caballi and T. equi infections
Direct examination of blood smears from adult horses showed an overall prevalence rate of 7.8%, with 7.2% due to T. equi, with parasitemias always below 0.1%. Only 1.0% of the horses had concurrent infections of T. equi and B. caballi. On the other hand, the prevalence rates obtained by the MRT-PCR were much higher, 59.7% for T. equi and 12.5% for B. caballi, with detection of concurrent infections in 8.6% of the horses.
The analysis of PCV values showed that only 5% of the horses had PCV values <25%, 26.4% had PCV values between 25 and 34%, and the great majority (68.6%) had PCV values >34%, within the normality range for horses. Similarly, the great majority of positive horses (70%) had normal PCV values (>34%) and, therefore, no significant correlations were seen between positivity in the MRT-PCR and PCV values. On the other hand, there was a significant correlation (p = 0.023) between a T. equi-positive blood smear and detection of infection by the MRT-PCR; however, the same association was not seen for B. caballi infections.
Seroprevalence
The overall seroprevalence was 91.0% for T. equi and 83.% for B. caballi, with high frequencies of titers of 1:40 and 1:640 for both parasites (Fig. 2). As far as fetus samples are concerned, no infection could be detected, neither by direct nor indirect methods, and therefore, intrauterine transmissions were not detectable in this study.
Molecular characterization
For the characterization of T. equi, 22 sequences from 11 different locations (Ipameri, GO; Jaíba, MG; Patos de Minas, MG; Ituiutaba, MG; Arenópolis, GO; São Sebastião do Paraíso, MG; Itajubá, MG; Montes Claros, MG; Santo Antônio da Barra, GO; Itajú do Colônia, BA; and Janaúba, MG) were compared within each other and with sequences available in the GenBank (access numbers: AF261824, AB015213). These comparisons showed an alignment score of 99%.
For the characterization of B. caballi, six sequences (from Jaíba, MG; Caetité, MG; Guaxupé, MG; Itajú do Colônia, BA; Itajubá, MG; and Quirinópolis, GO) were compared within each other and with sequences available in the GenBank (access numbers: AF092736, AB017700). Again, the alignment score was 97%. Thus, all sequences were identical to sequences available in the GenBank and had 100% identity to each other.
Discussion
In the present study, the occurrence of piroplasm infections was extensively investigated by blood-smear examination, MRT-PCR, and serology in horses from different locations in Brazil.
The results confirmed that both B. caballi and T. equi infections are widespread in the studied region, suggesting high levels of transmission, as demonstrated by the high percentages of positive animals and the high titers of specific antibodies in the IFAT. As expected under these circumstances, detection of infections in blood smears was very low, with parasitemias always below 0.1%, for both parasites. As all horses were adult and had probably been exposed to ticks and consequently to the parasites several times, the great majority had developed protective immunity and became chronically infected carriers. On the other hand, and despite the low patent parasitemia, the MRT-PCR developed in the present study proved to be efficient in detecting infected animals, undergoing either an acute or a chronic infection of B. caballi or T. equi.
The use of PCR or real-time PCR as a reliable tool to detect piroplasm infections has been well reported in the literature. Posnett and Ambrosio (1991) used DNA probes for the detection of B. caballi; Bahiruddin et al. 1999 used different sets of primers to determine the 16S-rRNA-gene of B. caballi and T. equi; nested PCRs were used by Nicolaiewsky et al. 2001 and Rampersad et al. 2003, and more recently a multiplex PCR was developed for simultaneous detection of B. caballi and T. equi in horses (Alhassan et al. 2005). However, the present study constitutes the first report of a MRT-PCR to detect the two horse parasites in Brazil and also the first molecular comparative characterization of Brazilian isolates. The PCR prevalence rates were high, particularly for T. equi, and comparable to those recently reported in a study carried out in Mongolia, also using a MRT-PCR but with different sets of primers (Rüegg et al. 2007).
However, despite the high rates of infections observed in the present study, no congenital transmission was detectable. Transplacental infection of foals with T. equi may result in either full-term stillbirths or the birth of live foals that develop clinical disease soon after birth (de Waal and van Heerden 1994), and neonatal deaths and abortions have been recently reported by Phipps and Otter 2004. However, most foals born from immune mares seem to be well protected against infections (Donnelly and Phipps 1982). Nevertheless, a recent study carried out on carrier mares in South Africa has shown, by the use of an oligonucleotide probe, high rates of transplacental transmission of T. equi to apparently healthy foals, occurring as early as 4 months of gestation (Allsopp et al. 2007). In that study, however, instead of blood, spleen samples were used to detect infection in fetuses. It is also important to emphasize that in the present study, the great majority of samples were collected from fetuses at a very early stage of pregnancy (less than 3 months), and this profile had possibly limited the detection of infection in fetal blood samples. Therefore, the epidemiological importance of congenital transmission of T. equi in horses in Brazil remains to be further investigated.
The high seroprevalence rate for T. equi observed in the present study correspond to those reported in previous epidemiological studies carried out with more limited numbers of samples in Brazil, in which prevalence rates ranged from 58 to 100% (Tenter and Friedhoff 1986; Ribeiro and Lima 1989; Cunha 1993 and Pfeifer Barbosa et al. 1995; Ribeiro et al. 1999; Heuchert et al. 1999). And although fewer reports have been previous published on prevalence of B. caballi, the rates were also high, ranging from 70 to 90% (Pfeifer Barbosa et al. 1995; Heuchert et al. 1999).
The dynamics of antibody production against B. equi and B. caballi in pregnant mares living under natural field conditions, as well as the efficiency of the passive transfer of antibodies via colostrum, have also been studied in the state of Minas Gerais (Passos et al. 1999). And, although the levels of antibodies in pregnant mares had no influence of on the passive transfer of antibodies, the passive transfer of B. caballi antibodies was less efficient than that of B. equi antibodies, indicating that young animals are at higher risk of acquiring this infection.
Although the two piroplasm infections were highly prevalent in horses, no significant correlations were detected between low PCV values and presence of infection (neither by serology nor direct detection). On the other hand, positivity in blood smears was significantly associated with detection of T. equi infection by the MRT-PCR (p = 0.02), indicating that the PCR could replace with advantages the direct detection through blood-smear examination for routine diagnosis of large numbers of samples.
Characterization of piroplasm isolates from Brazilian horses on a molecular basis was, so far, completely lacking. The present study is the first report in which isolates of B. caballi and T. equi from different areas of Brazil were compared within each other and with data available in the Genbank.
Diversity or even mutations were expected more in T. equi than in B. caballi isolates. The PCR for T. equi is based on detection of superficial proteins encoded by paralogous single-copy genes (Ueti et al. 2003), while the PCR for B. caballi amplificates rhoptria-proteins encoded by a gene containing more than two copies of the B. caballi genome. Therefore, sensitivity of the PCR for B. caballi is lower than that for T. equi (Ikadai et al. 1999).
The comparative analyses of sequences of the two piroplasms showed no differences within each species or among known sequences available and, therefore, no diversity was observed among the isolates present in the studied areas.
Transmission of piroplasms is usually influenced by the dynamics of vector populations, and these are directly influenced by climatic conditions. Thus, for large countries like Brazil, where climatic conditions are ranging from temperate to extremely tropical zones, the absence of diversity amongst isolates may be of great importance, favoring for instance the establishment of control measures, such as the use of vaccines, which would be equally efficient for the whole country.
Thus, the results from the present study confirmed the widespreadness of equine babesioses by B. caballi and T. equi in Brazil with homology among the isolates of both parasites. Further studies should be addressed to a better evaluation of congenital transmission under natural field conditions.
References
Alhassan A, Pumidonming W, Okamura M, Hirata H, Battsetseg B, Fujisaki K, Yokoyama N, Igarashi I (2005) Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Vet Parasitol 129:43–49
Allsopp MTEP, Lewis BD, Penzhorn BL (2007) Molecular evidence for transplacental transmission of Theileria equi from carrier mares to their apparently healthy foals. Vet Parasitol 148:130–136
Bahiruddin JB, Camma C, Rebelo E (1999) Molecular detection of Babesia equi and Babesia caballi in horse blood by PCR amplification of part of the 16S rRNA gene. Vet Parasitol 84:75–83
Battsetseg B, Lucero S, Xuan X, Claveria FG, Inoue N, Alhassan A, Kanno T, Igarashi I, Nagasawa H, Mikami T, Fujisaki K (2002) Detection of natural infection of Boophilus microplus with Babesia equi and Babesia caballi in Brazilian horses using nested polymerase chain reaction. Vet Parasitol 107:351–357
Böse R, Jorgensen WK, Dalgliesh RJ, Freidhoff KT, de Vos AJ (1995) Current state and future trends in the diagnosis of babesiosis. Vet Parasitol 57:61–74
Cunha CW (1993) Babesiose equina: Padronização da reação de imunofluorescência para sorodiagnóstico e levantamento epidemiológico em eqüinos puro sangue inglês (Thesis, Universidade Federal de Pelotas, RS, Brazil)
de Waal DT (1992) Equine piroplasmosis: a review Br Vet J 148:6–13
de Waal DT, van Heerden J (1994) In: Coetzer JAW, Thompson GR, Tustin RC (eds) Infectious diseases of livestock (with special reference to Southern Africa), vol 1. Oxford University Press, Cape Town, pp 295–304
Donnelly J, Phipps LP (1982) Evidence of maternal antibodies to Babesia equi and Babesia caballi in foals of seropositive mares. Equine Vet J 14:126–128
Friedhoff KT (1988) Transmission of Babesia. In: Ristic M (ed) Babesiosis of domestic animals and man. CRC, Boca Raton, pp 23–52
Ginther OS (1967) Reproductive biology of the mare—basic and applied aspects. Equiservices, Cross Plains, WI. 396
Guimaraes AM, Lima JD, Ribeiro MFB, Camargos ERS, Bozzi IA (1998a) Ultrastructure of sporogony in Babesia equi in salivary glands of adult female Boophilus microplus ticks. Parasitol Res 84:69–74
Guimaraes AM, Lima JD, Ribeiro MFB (1998b) Sporogony and experimental infection of Babesia equi by Boophilus microplus ticks. Parasitol Res 84:323–327
Heuchert CMS, Giulli V Jr, Athaide DF, Böse R, Friedhoff KT (1999) Seroepidemiologic studies on Babesia equi and Babesia caballi infections in Brazil. Vet Parasitol 85:1–11
Ikadai H, Xuan X, Igarashi I, Tanaka S, Kanemaru T, Nagasawa H, Fujisaki K, Suzuki N, Mikami T (1999) Cloning and expression of a 48-kilodalton Babesia caballi merozoite rhoptry protein and potential use of the recombinant antigen in an enzyme-linked immunosorbent assay. J Clin Microbiol 37:3475–3480
Nicolaiewsky TB, Richter MF, Lunge VR, Cunha CW, Delagostin O, Ikuta N, Fonseca AS, Silva SS, Ozaki LS (2001) Detection of Babesia equi (Laveran, 1901) by nested polymerase chain reaction. Vet Parasitol 101:9–21
Passos LMF, Ribeiro MFB, Anderegg PI, Böse R (1999) Serological diagnosis of Babesia equi and B. caballi in pregnant mares. Arq Bras Med Vet Zootec 51:527–530
Pfeifer Barbosa I, Böse R, Peymann B, Friedhoff KT (1995) Epidemiological aspects of equine babesioses in a herd of horses in Brazil. Vet Parasitol 58:1–8
Phipps LP, Otter A (2004) Transplacental transmission of Theileria equi in two foals born and reared in the United Kingdom. Vet Rec 154:406–408
Posnett ES, Ambrosio RE (1991) DNA Probes for the detection of Babesia caballi. Parasitol 102:357–365
Rampersad J, Cesar E, Campell MD, Samlal M, Ammons D (2003) A field evaluation of PCR for the routine detection of Babesia equi in horses. Vet Parasitol 114:81–87
Ribeiro MFB, Lima JD (1989) Diagnóstico sorológico de babesiose equine por Babesia equi em Minas Gerais. Seminário Brasileiro de Parasitologia Veterinária. IV, Bagé, p 111
Ribeiro MFB, Saito JF, Pimentel PV (1995) Babesiose equina. I. Primo-infecção de potros em área endêmica. Arq Bras Med Vet Zootec 47:641–647
Ribeiro MFB, Costa JO, Guimaraes AM (1999) Epidemiological aspects of Babesia equi in horses in Minas Gerais, Brazil. Vet Res Commun 23:385–390
Roby TO, Anthony DW (1963) Transmission of equine piroplasmosis by the tropical tick Dermacentor nitens (Neumann). J Am Vet Med Assoc 142:768–769
Rüegg SR, Torgerson P, Deplazes P, Mathis A (2007) Age-dependent dynamics of Theileria equi and Babesia caballi infections in southwest Mongolia based on IFAT and/or PCR prevalence data from domestic horses and ticks. Parasitology 134:939–947
Tenter AM, Friedhoff KT (1986) Serodiagnosis of experimental and natural Babesia equi and B. caballi infections. Vet Parasitol 20:49–61
Ueti MW, Palmer GH, Kappmeyer LS, Scoles GA, Knowles DP (2003) Expression of equi merozoite antigen 2 during development of Babesia equi in the midgut and salivary gland of the vector tick Boophilus microplus. J Clin Microbiol 41:5803–5809
Uilenberg G (2006) Babesia-a historical overview. Vet Parasitol 138:3–10
Acknowledgments
The authors thank the personnel and the veterinarians at the slaughterhouse Pomar in Araguari for allowing the collection of samples, Mr. Ricardo Canesso Dalla Rosa and Ms. Ana Paula Ferreira for the technical assistance in the laboratory, Ms. Denise Guethlin for assistance in the statistical analysis, the Brazilian agency CAPES (Project 182/04) and the German agency DAAD for the financial support for exchanging scientists and students, and the Brazilian National Council for Scientific and Technological Development (CNPq) for awarding scholarships. The experiments carried out in the present study comply with the current laws of Brazil and Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heim, A., Passos, L.M.F., Ribeiro, M.F.B. et al. Detection and molecular characterization of Babesia caballi and Theileria equi isolates from endemic areas of Brazil. Parasitol Res 102, 63–68 (2007). https://doi.org/10.1007/s00436-007-0726-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0726-1