Abstract
Egypt is the world’s ninth-largest fish producer with a total production of 1.5 billion tons per year, and farmed fishes comprise almost 79.6% of the total production. Massive mortalities in market-sized farmed fish (freshwater and marine species) were documented all over fish farms in Egypt leading to serious economic losses. The poor biosecurity practices and awareness among smallholder fish farmers accompanied with a long period of temperature fluctuation may predispose farmed fish to be simultaneously coinfected with multiple pathogens. Moribund fishes usually exhibited various septicemic clinical signs and post-mortem lesions indicating that one or more septicemic bacterial pathogens were involved in these outbreak reports. Therefore, rapid and accurate identification of pathogens in the asymptomatic fish population is important for preventing the occurrence of infectious diseases and protecting consumers from seafood-borne illnesses. Molecular techniques provide sensitive, quick, and accurate data for identifying specific pathogens without the need for time-consuming traditional techniques. In addition, the development of cost-effective molecular techniques facilitates their wide implementation in routine clinical diagnostic approaches. Molecular diagnostic methods are useful for simultaneously identifying multiple bacterial pathogens that were challenging to recognize by commercial biochemical techniques. Genetic testing procedures could determine the genetic diversity between closely related strains at the subspecies level. Consequently, these techniques are required for the development of detecting methods for specific strains and for epidemiological investigations in bacterial diseases. This review documented a variety of molecular techniques, including amplification of nucleic acids, polymerase chain reaction (PCR), nested PCR, real-time PCR, multiplex PCR, loop-mediated isothermal amplification (LAMP), DNA microarrays, and nucleotide sequencing assays, that are commonly used to identify fish pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mass mortalities in aquaculture
Fish represented about 38% of animal protein intake in Egypt. However, Egypt’s self-sufficiency in fish production declined from 82.9 to 67.3% (Abdelsalam et al. 2021). To fill this gap, the Egyptian government reveals and finances several fish farming projects and encourages the private sector to invest in aquaculture. Egyptian aquaculture contributed significantly 79.6% to the overall fisheries production. Most Egyptian fish farms are situated in rural provinces which explained the importance of rapid diagnostic kits that can be applied to on-farm sites (Ali et al. 2020). Egypt has experienced eruptions of mass kills caused by various septicemic bacterial pathogens that involved cultured marine and freshwater fishes (Table 1). Fish mass kills may be referred to as unexpected, sudden, and acute mass mortalities among the wild and farmed fish population over a short time (El-Mezayen et al. 2018). Because of the socio-economic significance of the Egyptian aquaculture sector, greater emphasis was directed to the rapid identification of pathogens affecting cultured fish (Eissa et al. 2021a). A survey showed that 37% of fish farms located in Ash-Sharqia, Beheira, and Kafr El-Shaikh Governorates experienced mass mortality with severe economic losses (Fathi et al. 2017). Another survey indicated that 78.8% of fish farms suffered from mass mortality. However, the suspected etiologies remain unidentified (Sherif et al. 2020; 2021a; Ali et al. 2020). Several studies denoted that fish mass mortalities might occur due to the invasion of septicemic bacterial pathogens in susceptible fish under adverse aquatic environmental conditions. It was found that fish mass mortality events are highly correlated to the co-occurrence of diverse bacterial pathogens in the same fish host (Carella et al. 2020). The diagnosis of coinfection is difficult and receives little consideration due to the complexity of the interaction among involved pathogens (Carella et al. 2020).
Bacterial coinfections
Bacterial diseases affecting fish are one of the most challenging issues threatening fish’s life in the wild and captivity (Elgendy et al. 2017; 2021; EI-Jakee et al. 2020; El-Adawy et al. 2020). Bacterial infections seldom occur as a single trigger case, while mixed infections are more likely (Moustafa et al. 2015). Bacterial pathogens cause infections in fish, concomitantly with other infectious agents, including viruses, mycotic, and parasitic agents (Austin 2019). Coinfections occur as two or more genetically different microbial pathogens infect the same host simultaneously, either as a primary or secondary concurrent infection (Abdel-Latif and Khafaga 2020). Coinfection can change the host’s susceptibility to specific pathogens. It can significantly affect the dynamics of fish disease outbreaks by amplifying the severity, duration, and progression of infection. In addition, coinfection can affect the clinical outcomes and pathological effects in the affected host (Elgendy et al. 2016).
The impact of concurrent infectious pathogens on each other could have synergistic or antagonistic effects (Bradley and Jackson 2008). In synergistic coinfection, the primary pathogen suppresses the infected host’s immune system, allowing the secondary pathogen to upsurge disease severity and fish mortalities (Dong et al. 2016). Aeromonas hydrophila is one of such listed pathogens which acts synergistically with tilapia lake virus (TilV) to increase mortality and worsen the disease severity in tilapia (Nicholson et al. 2017, 2020). On the other hand, antagonistic coinfection occurs when the primary pathogen modifies the immunological reaction and consequently delays the secondary pathogen (Kotob et al. 2016). Unfortunately, bacterial coinfections may be considered evidence of contamination and accordingly cause complications during the diagnostic procedures, due to the existence of more than one pathogen (Austin 2019). In addition, bacterial coinfection could retard the immunoprophylaxis process and diminish the vaccination efficiency in moribund fish (Figueroa et al. 2017). Therefore, the identities of the real pathogens standing behind several coinfections have not been reported in many cases.
Septicemic bacterial infections may manifest in various ways, including acute, sub-acute, and chronic types. Infected fish usually exhibit hemorrhages at the base of the fins, around the head, and the belly. Darkening, skin erosions, ulcers, and corneal opacity are all common in moribund fish (Roberts 2012). Internal organs, including the spleen, liver, and kidney, are enlarged, congested, and liquefied. Moreover, paleness of gills, ascites, and petechiation of visceral and parietal peritoneum are commonly seen. Circulatory, proliferative, necrotic, and degenerative alterations are often noticed in tissues from infected fish (Roberts 2012; Mahmoud et al. 2016). However, such gross lesions could not be differentiated between suspected bacterial pathogens. Edwardsiella ictaluri infections in farmed tilapia with visceral white spots were misdiagnosed as Francisella (Dong et al. 2019).
Mobile elements and virulence
Pathogenic pathways of septicemic bacterial infections are triggered by several harmful extracellular products (ECPs) (Baffone et al. 2001). These ECPs are the primary causes of pathological alterations and mortality by destroying essential elements of both the circulatory and immune systems of fish (Li et al. 2003). Several virulence genes are frequently linked with mobile genetic elements, such as insertion sequences (IS) that enable recipient bacterial species to acquire foreign virulence genes from closely related bacterial pathogens. Insertion sequences could establish compound transposons by bordering virulence genes and promoting horizontal gene transfer. Furthermore, IS plays a significant role in rearranging genetic materials such as deletion, translocation, inversion, and duplication (Abdelsalam et al. 2009; 2010; 2015). The insertion and deletion mutations in virulence genes might associate with the development of bacterial pathogenesis and might help recipient bacterial virulence (Igbinosa et al. 2012). On the other hand, it was found that the propagation of insertion sequences among numerous streptococcal isolates collected from diseased fishes proposed that horizontal gene transfer and recombination events could happen in these bacterial species. It was suggested that insertion sequences might be incriminated in transferring the streptococcal pyrogenic exotoxin G gene (spegg) from Streptococcus dysgalactiae ssp. equisimilis (pig isolates) to S. dysgalactiae ssp. dysgalactiae (fish isolates) through transposons, signifying that the insertion sequence might contribute to bacterial virulence (Abdelsalam et al. 2010; 2017).
Limitations of traditional techniques in bacterial identification
The traditional identification of bacterial diseases is depending mainly on agar cultivation and the phenotypic and serological characteristics of the pathogen (Altinok and Kurt, 2003). The culture-dependent assays used in the identification of bacteria relied on the isolation of bacteria in pure dense growth. The success of these techniques depends upon numerous factors: proper selection of diseased tissue, proper selection of specific and general media, appropriate incubation conditions, and appropriate selection of meaningful colonies for further study (Austin, 2019). Moreover, the acquisition of different pure culture colonies could reflect the retrieval of primary and secondary invaders, or even bacterial contaminants from moribund fish. The bacteriological traditional examination could not identify the possible bacterial sequence infection whereby the primary organism that initiates the infection could be masked by others. To date, pure colonies could be served for reference purposes, epizootiology protocols, determination of pathogenicity features, and determination of antibiotic susceptibility patterns. Culturing-dependent techniques require a variety of media, incubation conditions, and the excellent expertise of microbiologists to select the proper colonies for purification. Therefore, this method is a time-consuming diagnostic tool, representing a major challenge when the real thrust is on controlling fish diseases (Austin 2019).
Principal traditional bacteriological tests frequently comprise colony shape, Gram stain, a motility test, catalase and/or oxidase reaction, and oxidation/fermentation of glucose (Cai et al. 2014). These tests might be generally enough to identify the recovered bacteria to the genus level in Table 2. However, the routine biochemical techniques failed to differentiate among Y. ruckeri, E. tarda, and E. ictaluri (Danley et al. 1999). Several commercial biochemical kits are made available that are not specifically intended to be used with aquatic pathogens; however, many fish pathologists have used them after some adaptations (Farzadnia and Naeemipour 2020). The most common available kits involve the Vitek® system, API-20E, API-20NE, API-20Strep, API-50L, API-ZYM, API-50CH, Rapid-ID32 systems, BioMérieux, Biolog MicroPlates, Biolog-GP, Biolog-GN (Biolog, Inc.), and the BBL® Enterotube™ II system (Becton, Dickinson) (Austin 2019). These kits are considered labor-intensive, time-consuming, and not able to identify several bacterial pathogens in aquaculture. In some cases, API-20E could not differentiate between Yersinia ruckeri and Hafnia alvei (Danley et al. 1999). In addition, A. dhakensis was primarily misidentified as A. hydrophila via the VITEK-2 kit, while the sequencing process confirmed the identity of the isolate as A. dhakensis (Haung et al. 2020). Moreover, the API-20E yielded false positive and negative reactions for different sugars’ fermentation, Voges Proskauer (VP), lysine decarboxylase (LDC), gelatinase, and citrate reactions. Furthermore, the API-20E failed to identify 59% of A. salmonicida isolates and 83% of Y. ruckeri strains. Additionally, Vibrio anguillarum, A. sobria, and A. caviae strains were usually misidentified as A. hydrophila by using the API-20E. P. piscicida isolates were misidentified as Ps. fluorescens or Ps. putida by using the API-20E (Santos et al. 1993). These traditional methods might cause misdiagnosis due to the phenotypic variability between different strains from the same species, and the inconsistency of biochemical results due to the adjustment in the manufacturer protocol to adopt aquatic pathogens (Cunningham 2002). The culture condition of recovered bacteria might also affect the accuracy of these methods (Abdelsalam et al. 2017).
Moreover, some isolates of Streptococcus spp. failed to be identified using the Rapid Strep system (Abdelsalam et al. 2017). Biochemical diagnostic tests, including API 20 Strep, Rapid ID 32 Strep, BioMérieux Vitek, the ATB Expression System, and the MicroScan WalkAway System, misidentified S. iniae as S. dysgalactiae subsp. equisimilis. This misidentification took place because S. iniae was not incorporated in the databases of any of the automated devices (El Aamri et al. 2010).
On the other hand, immuno-serological assays depend mainly on the specific reaction of commercial monoclonal and polyclonal antibodies against the particular antigens in the sample, or vice versa. These techniques include western blot and dot blot (blotting techniques), immunohistochemistry (IHC), enzyme-linked immunosorbent assay (ELISA), latex agglutination, whole-cell agglutination, immune-India ink technique, and fluorescent antibody techniques (FAT and IFAT). However, these methods sometimes fail to identify the bacterial agent due to the bacterial concentration within the specimen being lower than the sensitivity threshold of these techniques, and in some cases, processing faults of the sample might cause antigenic alterations and the pathogens are no longer recognized by the antibody (Adams and Thompson 2011). Nevertheless, serological techniques are still used for the identification of fish diseases in laboratory and field conditions (Austin 2019). These limitations of conventional methods have resulted in the expansion in the usage of molecular techniques depending upon their higher specificity and sensitivity.
Molecular techniques for identifying fish pathogens
Molecular diagnostic methods are more accurate and faster for identifying septicemic bacterial pathogens compared to traditional techniques. Rapid identification of fish diseases would help commercial aquaculturists and fish pathologists to design effective disease control strategies on their farms (Farzadnia and Naeemipour 2020). Precise diagnosis of bacterial pathogens permits using the appropriate antimicrobials and consequently preventing the occurrence of antibiotic resistance in fish farms (Austin 2019). In addition, quick identification of pathogenic bacteria in asymptomatic or carrier fish would help in the development of biosecurity programs. Moreover, the abundance and the concentration of pathogenic bacteria in the aquatic environment and the fish could be also monitored during the production cycle (Austin 2019). Furthermore, detection of pathogens in the aquatic environment between fish harvesting and re-stocking could be beneficial in disease outbreak prevention (Farzadnia and Naeemipour 2020).
Polymerase chain reaction (PCR) is one of the most common molecular techniques which is widely used for the rapid detection of fish pathogenic bacteria. Other PCR-based diagnostic assays are real-time (RT) PCR, nested PCR, reverse-transcription PCR, and multiplex PCR which are applied for the concurrent and quantitative detection of a variety of heterogeneous pathogenic bacteria. Genomic DNA extraction from the sample is typically the keystone of molecular techniques. Extracted DNA can be subsequently amplified using specific primers by PCR for diagnostic purposes and can be further analyzed by random amplification of polymorphic DNA (RAPD) and restriction fragment length polymorphism (RFLP), or even probed by DNA hybridization (Austin 2019; Cai et al. 2014). PCR assays can identify about 2 cells of Aeromonas salmonicida, while 16S rRNA sequencing can detect 103 colony-forming units of A. salmonicida (Austin 2019). The 16S rRNA gene is the most common universal gene used for the detection of all bacterial species. This gene comprises species-specific regions that are used to discriminate between various bacterial species (Janda and Abbott 2007).
On the other hand, isothermal techniques can detect pathogenic bacteria under isothermal conditions without using expensive thermocycler equipment. These techniques were developed for the rapid diagnosis of fish pathogenic bacteria. Loop-mediated isothermal amplification (LAMP) is one of these more advanced tools than PCR (Table 3). LAMP assays use 4–6 primers; therefore, they are more specific than traditional PCR (Adams and Thompson 2011). In addition, LAMP assays have the following: high-speed detection (less than 1 h), high efficiency, cost-efficiency, simplicity to use, simple analysis, and detection of various pathogenic bacteria in aquaculture. Finally, the DNA microarray method has also been used in the concurrent identification of several pathogenic bacteria (Farzadnia and Naeemipour 2020). Compared to microarray and PCR-based assays, DNA sequencing assays can be directly applied to detect nonculturable pathogenic bacteria by amplifying and sequencing genes using universal primers.
Polymerase chain reaction
PCR is the most common DNA-based diagnostic assay used continually in the detection of fish bacterial pathogens. PCR technique can amplify millions of copies of specific gene sequences from a small amount of DNA using specific primers and DNA polymerase. The primers are annealed in a specific part of DNA to determine where DNA polymerase starts and stops copying the DNA. PCR cycling includes three stages: denaturation or separation of the two strands of the DNA at 96 °C, followed by annealing of primers at specific regions on DNA strands, and finally ended with the extension of DNA by DNA polymerase at 72 °C (Farzadnia and Naeemipour 2020). PCR is usually carried out in 25–40 cycles and should yield the expected size of the amplicons (Abdelsalam et al. 2017). The size of amplicons could be detected through gel electrophoresis (Eissa et al. 2021a, b). The PCR product may be used for different molecular procedures including sequencing, cloning, and detecting pathogens (Farzadnia and Naeemipour 2020). Consequently, PCR can identify nonculturable pathogenic bacteria. The sensitivity and specificity of PCR are higher than of other traditional methods; however, PCR might yield non-specific bands or yield unclear bands due to insufficient PCR products. This problem can be overcome by using touch-down PCR and hot-start PCR.
In the field of diagnosis of fish bacterial diseases, this technique is used for the detection of A. hydrophila, A. salmonicida, V. anguillarum, Y. ruckeri, L. garvieae, P. damselae, and S. dysgalactiae (Abdelsalam et al. 2017; Eissa et al. 2021a, b). However, the main disadvantage of PCR is that this method still depends on bacterial culture for genetic typing. In addition, false negative results might occur due to several factors including inappropriate sample collection, degradation of nucleic acids, an inadequate quantity of specimens, specimen mix-up, contaminants in the lab, and contaminant samples with normal flora (Narayana et al. 2018). In Egypt, PCR was used to identify A. hydrophila and V. parahaemolyticus coinfection in moribund-farmed striped mullet Mugil cephalus (El-Son et al. 2021).
Nested PCR
For the sake of increasing the sensitivity and specificity of the PCR reaction, two sets of different primer pairs were applied in two successive PCR amplifications. The nested PCR could use less target DNA and consequently could amplify low-abundance genes (Liop et al. 2000). Briefly, amplicons yielded in the 1st amplification are exposed to a 2nd amplification. This technique was described for the first time by Kamolvarin et al. (1993). The main drawback of nested PCR is the high probability of Eppendorf contamination due to repeated opening of the vessel to carry out the two amplification cycles. This could have resulted in a large rise of false positives. Nested PCR allowed the identification of R. salmoninarum, V. vulnificus, A. hydrophila, E. tarda, S. iniae, and P. damselae at very low densities of bacterial infection: 3–4 bacterial cells per gram tissues of fish (Farzadnia and Naeemipour 2020). In Egypt, nested PCR has been successfully applied in the diagnosis of mixed infection of Streptococcus and Myxobolus tilapiae coinfections in moribund Nile tilapia (Eissa et al. 2021b).
Multiplex PCR
Multiplex PCR can simultaneously target and amplify several specific genes from different bacterial pathogens using multiple primer pairs and yield different sizes of specific DNA products. This technique was described for the first time in 1988 as a possible technique to distinguish the deletions in the dystrophin gene (Chamberlain et al. 1988). This technique has high accuracy and is cost-effective and time-saving. In addition, it decreases pipette errors (Ador et al. 2021). This method has been applied for concurrent identification of three different pathogenic bacteria (A. hydrophila, F. columnare, and E. ictaluri), five different pathogenic bacteria (Y. ruckeri, A. salmonicida subsp. salmonicida, A. hydrophila, R. salmoninarum, and F. columnare) (Farzadnia and Naeemipour 2020), and four different pathogenic bacteria (V. parahaemolyticus, V. cholerae, V. alginolyticus, and V. vulnificus) (Adams and Thompson 2011). This assay was used for the simultaneous identification of three different pathogens, namely A. salmonicida, F. psychrophilum, and Y. ruckeri (Del Cerro et al. 2002). The mPCR was also applied for simultaneous recognition of four different pathogens, namely F. psychrophilum, L. garvieae, Ps. putida, and Ps. aeruginosa (Altinok and Kurt 2003). In addition, multiplex PCR was used to simultaneously identify four different Gram-positive pathogens: S. parauberis, S. iniae, L. garvieae, and S. difficilis (Mata et al. 2004).
Multiplex PCR allowed the identification of Vibrio, Aeromonas, Streptococcus, and Edwardsiella at very low densities of the bacterial count of 100 CFU in fish tissues and 50 CFU in culture. However, a multiplex PCR could not incorporate more than six primer pairs to avoid cross-reactions between target DNA and primers (Austin 2019). However, the main disadvantage of this method is that the standardization of this technique may need numerous trials, and self-inhibition among primer pair sets could happen (Ador et al. 2021). In Egypt, multiplex PCR was used to identify A. hydrophila and Y. ruckeri coinfecting farmed fish (El-Hady and Ahmed 2014).
Real-time PCR
Real-time PCR or quantitative PCR allows determining the number of yielded amplicons depending upon the binding of SYBR®Green dyes to double-stranded DNA, and then emitting fluorescence through the excited fluorophore. RT-PCR detects the targeted DNA amplification in the progression of the PCR, not at the termination as in traditional PCR. The sensitivity and specificity of real-time PCR are very high and can determine very low levels of DNA in different tissue samples. The RT-PCR method is repeated 25–50 times and involves a series of temperature fluctuations (Austin 2019). The period and the suggested temperature used for every cycle in RT-PCR are based on a diversity of factors, such as the annealing temperature of the primers, the concentration of divalent ions, deoxyribonucleotides (dNTPs) in the reaction, and the enzyme used to synthesize the DNA (Austin 2019). This technique enables highly accurate recognition of low-copy DNA targets and does not require gel documentation. The major advantage is the ability to quantify the DNA or RNA template existing in the specimen (Tom et al. 2004). RT-PCR technique requires higher technical skills and expertise for developing the assay. In addition, kits of RT-PCR do not exist for different genes of causative agents, and the standardized procedures are limited.
Therefore, the assay is an expensive test and hard to scale. Although this method could indicate the incidence of pathogenic DNA material during infection, it cannot determine whether the host is infected or not. Real-time PCR is used for the identification and quantification of pathogenic bacteria in fish such as A. salmonicida and S. parauberis (Austin 2019). The qPCR showed 99.2% accurate sensitivity in detecting Francisella noatunensis subsp. orientalis widespread in the farmed O. niloticus (Assis et al. 2017). The multiplex qPCR was successfully applied to identify A. salmonicida, V. anguillarum, and T. maritimum concurrent infections (Chapela et al. 2018). The results were consistently confirmed in all methodological replicates, proving the high specificity of this technique to distinguish coinfection situations or natural contamination problems (Chapela et al. 2018). In Egypt, real-time PCR was applied to detect coinfection of tilapia lake virus and different Aeromonas spp. coinfecting Nile tilapia (Nicholson et al. 2017).
Loop-mediated isothermal amplification
LAMP is a cost-effective, simple, fast, and highly specific method for amplification of nucleic acid. This tool uses six different primer sequences and a Bst DNA polymerase to target specific genes. This polymerase enzyme has the strong activity of 5′–3′ DNA polymerase and strand displacement but lacks 5′–3′ exonuclease activity. This enzyme is used in the LAMP amplification reaction. The amplified products are stem-loop DNA structures with several inverted repeats of the target and cauliflower-like structures with multiple loops, yielding > 500 mg/mL of PCR products (Nagamine et al. 2001).
LAMP is highly specific and does not require sophisticated instruments like a thermocycler. In addition, the accumulation of magnesium pyrophosphate insoluble salt in the final mixture reaction resulted in the development of an observable white precipitate (Farzadnia and Naeemipour 2020). Thus, positive and negative reactions could be differentiated by the naked eye. LAMP techniques have been applied for the identification of E. tarda, V. anguillarum, and S. iniae (Austin 2019). Triplex LAMP was applied to simultaneously detect V. alginolyticus, V. anguillarum, and V. harveyi, using three sets of primers in a single reaction with very higher sensitivity compared with traditional PCR (Austin 2019). Finally, a real-time fluorogenic LAMP assay was successfully applied to identify V. parahaemolyticus, V. anguillarum, N. seriolae, V. alginolyticus, Ps. putida, V. harveyi, V. vulnificus, S. iniae, V. fluvialis, and V. rotiferianus within 30 min (Zhou et al. 2014).
Universal DNA sequencing
Molecular identification of retrieved bacterial isolates was carried out by targeting specific genes. Several genes could be used in bacterial identification. However, amplification of the 16S rRNA gene followed by sequencing is the most common method we often used in the identification of the seafood-borne pathogen (Janda and Abbott 2007; Naas et al. 2019). The massive DNA sequencing method can be used to simultaneously amplify mixed infections without any error. Thus, the most common housekeeping genetic marker 16S rRNA gene is used to study bacterial taxonomy and phylogeny because it exists in almost all bacteria and its function does not change over time, signifying that random changes in the DNA sequence are an accurate parameter of bacteria evolution. In addition, the size of the 16S rRNA gene is 1500 bp which is considered to be enough for bioinformatics resolutions (Azwai et al. 2016).
Bacterial 16S rRNA genes harbor 9 “hypervariable regions” (V1–V9) that display significant sequence diversity among diverse bacterial species. V1 is the most suitable for distinguishing Staphylococcus aureus and coagulase-negative Staphylococcus sp. V2 and V3 are the best to differentiate among all bacterial species to the level of bacterial genus excluding closely correlated Enterobacteriaceae (Chakravorty et al. 2007). The validity of these techniques is based strongly on the selected primers (Fig. 1). The universal bacterial primer pair that can amplify 464 bp was experimentally assessed by matching the taxonomic distribution of the 16S rDNA sequences with fragments of 16S rDNA deposited in the GenBank database (Klindworth et al. 2013). Several studies in Egypt have employed the sequencing of 16S rRNA in the diagnosis of different fish bacterial pathogens such as S. agalactiae and S. dysgalactiae coinfection in farmed red tilapia (Abdelsalam et al. 2017).
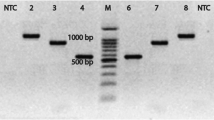
Sequencing steps of 16S rRNA gene for A. hydrophila identification using the universal primer pair of pathogenic bacteria. Suspected samples were prepared for bacteriological analysis using a selective medium agar. DNA is extracted from purified colonies and PCR reactions are performed. ABI PRISM.® 3130 Genetic Analyzer amplicon sequence chromatogram of 16S rRNA gene from A. hydrophila and then analyzed by suitable bioinformatics software (Okonechnikov et al. 2012)
DNA microarrays
These methods comprise numerous DNA probes immobilized on glass slides and are applied for the concurrent identification of several microbes in a single hybridization assay (Austin 2019). Microarrays are very sensitive and specific tools used for concurrent diagnosis of five different bacterial pathogens: A. salmonicida, V. parahaemolyticus, V. anguillarum, V. vulnificus, and P. damselae subsp. damselae. In addition, microarray was also used for simultaneous identification of eight different pathogens normally encountered in fish farms, namely A. hydrophila, E. tarda, F. columnare, L. garvieae, P. damselae, Ps. anguilliseptica, S. iniae, and V. anguillarum (Chang et al. 2012). This method was also used to differentiate between different types of bacterial pathogens infecting cultured fish, i.e., V. harveyi, V. parahaemolyticus, V. alginolyticus, V. cholerae, V. anguillarum, A. hydrophila, S. iniae, and Nocardia seriolae (Shi et al. 2012).
Although DNA microarrays are very useful for the simultaneous detection of thousands of fish pathogens in one step, these tools are expensive and usually need large amounts of nucleic acid. Microarrays could be grouped into four main categories depending on the represented genes on the assay: (1) phylogenetic oligonucleotide arrays, used to identify specific pathogens and compare their genetic interrelationship with other microbial groups depending on a conserved gene marker including 16S rRNA gene; (2) functional gene arrays, used to provide information about the functional genes incorporated in microbial populations, physiological procedures, and antibiotic resistance; (3) community genome arrays, used to describe microbial isolate by investigating the whole genomic DNA of cultivated bacteria; and (4) metagenomic arrays, used to identify microbes with no previous sequencing data by using probes designed from environmental DNA itself (Tang and Stratton 2006).
Conclusion
Bacterial coinfections are often recorded in wild and farmed fish when more than one bacterial pathogens coinfect fish. In Egypt, mixed infections may lay behind the different clinical signs and are responsible for difficulties in diagnosis and control régimes in closed aquatic environments. Therefore, dependence on traditional methods only for diagnosis may mask the identity of real causes.
On the other hand, molecular methods have improved the ability to identify bacterial pathogens. The whole-genome sequencing of microorganisms has made significant progress in understanding their biology and makes it possible to increase the specificity, sensitivity, and speed of diagnosis by using these nucleic acid techniques. These techniques also provide a way to examine the connections between the genotype and phenotype of different micro-agents. These advanced techniques may support epidemiological investigations and help to pinpoint the origins of illness outbreaks or the existence of infections. Therefore, these genetic technologies can be used frequently in the quest for better ways to identify and manage fish infections and understand the spread of infectious diseases in fish. Unfortunately, there are few diagnostic wet laboratories in Egypt that use these techniques regularly. In addition, there are several problems to be fixed before using these tests in aquatic laboratories, such as the availability of specific equipment, cost-effectiveness for regular operation, personnel training, accuracy, and reproducibility. Currently, PCR-based techniques are widely used; however, alternative technologies needed to be implanted in laboratories in the coming years as sequencing devices. Therefore, this review shed the light on the importance of popular molecular tools in the thrust of identification of bacterial pathogens in farmed fish.
Data availability
All required data are included in this article.
Code availability
Not applicable.
References
Abdel-Latif HMR, Khafaga AF (2020) Natural co-infection of cultured Nile tilapia Oreochromis niloticus with Aeromonas hydrophila and Gyrodactylus cichlidarum experiencing high mortality during summer. Aquac Res 51:1880–1892
Abdelsalam M, Chen SC, Yoshida T (2009) Surface properties of Streptococcus dysgalactiae strains isolated from marine fish. Bull Eur Assoc Fish Pathol 29:15–23
Abdelsalam M, Chen SC, Yoshida T (2010) Phenotypic and genetic characterization of Streptococcus dysgalactiae strains isolated from fish collected in Japan and other Asian countries. FEMS Microbiol Lett 302:32–38
Abdelsalam M, Eissa AE, Chen SC (2015) Genetic diversity of geographically distinct Streptococcus dysgalactiae isolates from fish. J Adv Res 6(2):233–238
Abdelsalam M, Elgendy MY, Shaalan M, Moustafa M, Fujino M (2017) Rapid identification of pathogenic streptococci isolated from moribund red tilapia (Oreochromis spp.). Acta Vet Hung 65:50–59
Abdelsalam M, Ewiss MAZ, Khalefa HS, Mahmoud MA, Elgendy MY, Abdel-Moneam DA (2021) Coinfections of Aeromonas spp., Enterococcus faecalis, and Vibrio alginolyticus isolated from farmed Nile tilapia and African catfish in Egypt, with an emphasis on poor water quality. Microb Pathog 160:105213
Adams A, Thompson KD (2011) Development of diagnostics for aquaculture: challenges and opportunities. Aquac Res 42:93–102
Ador MAN, Haque MDS, Paul SI, Chakma J, Ehsan R, Rahman A (2021) Potential application of PCR based molecular methods in fish pathogen identification: a review. Aquac Studies 22(1):621. https://doi.org/10.4194/2618-6381/AQUAST621
Ali SE, Jansen MD, Mohan CV, Delamare-Deboutteville J, Charo-Karisa H (2020) Key risk factors, farming practices and economic losses associated with tilapia mortality in Egypt. Aquaculture 527:735438
Altinok İ, Kurt İ (2003) Molecular diagnosis of fish diseases: a review. Turk J Fish Aquat Sc 3:131–138
Assis GBN, de Oliveira TF, Gardner IA, Figueiredo HCP, Leal CAG (2017) Sensitivity and specificity of real time PCR and bacteriological culture for francisellosis in farm raised Nile tilapia (Oreochromis niloticus L.). J Fish Dis 40(6):785–795
Austin B (2019) Methods for the diagnosis of bacterial fish diseases. Mar Life Sci Technol 1:41–49
Azwai SM, Alfallani EA, Abolghait SK, Garbaj AM, Naas HT, Moawad AA, Gammoudi FT, Rayes HM, Barbieri I, Eldaghayes IM (2016) Isolation and molecular identification of Vibrio spp. by sequencing of 16S rDNA from seafood, meat and meat products in Libya. Open Vet J 6:36–43
Baffone W, Citterio B, Vittoria E, Casaroli A, Pianetti A, Campana R, Bruscolini F (2001) Determination of several potential virulence factors in Vibrio spp. isolated from sea water. Food Microbiol 18:479–488
Bradley JE, Jackson JA (2008) Measuring immune system variation to help understand host-pathogen community dynamics. Parasitology 135:807–823
Byers HK, Gudkovs N, Crane MSJ (2002) PCR-based assays for the fish pathogen Aeromonas salmonicida. I. Evaluation of three PCR primer sets for detection and identification. Dis Aquat Org 49:129–138
Cai HY, Caswell JL, Prescott JF (2014) Nonculture molecular techniques for diagnosis of bacterial disease in animals: a diagnostic laboratory perspective. Vet Pathol 51:341–350
Carella F, Antuofermo E, Farina S, Salati F, Mandas D, Prado P, Panarese R, Marino F, Fiocchi E, Pretto T, De Vico G (2020) In the wake of the ongoing mass mortality events: co-occurrence of Mycobacterium, Haplosporidium and other pathogens in Pinna nobilis collected in Italy and Spain (Mediterranean Sea). Front Mar Sci 7:48. https://doi.org/10.3389/fmars.2020.00048
Chakravorty S, Helb D, Burday M, Connell N, Alland D (2007) A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J Microbiol Methods 69:330–339
Chamberlain JS, Gibbs RA, Ranier JE, Nguyen PN, Caskey CT (1988) Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 16(23):11141–56. https://doi.org/10.1093/nar/16.23.11141
Chang CI, Hung PH, Wu CC, Cheng TC, Tsai JM, Lin KJ, Lin CY (2012) Simultaneous detection of multiple fish pathogens using a naked-eye readable DNA microarray. Sensors 12:2710–2728
Chapela MJ, Ferreira M, Varela C, Arregui L, Garrido-Maestu A (2018) Development of a multiplex real-time PCR method for early diagnosis of three bacterial diseases in fish: a real-case study in trout aquaculture. Aquaculture 496:255–261
Cunningham CO (2002) Molecular diagnosis of fish and shellfish diseases: present status and potential use in disease control. Aquaculture 206:19–55
Danley ML, Goodwin AE, Killian HS (1999) Epizootics in farm-raised channel catfish, Ictalurus punctatus (Rafinesque), caused by the enteric red mouth bacterium Yersinia ruckeri. J Fish Dis 22:451–456
Del Cerro A, Marquez I, Guijarro JA (2002) Simultaneous detection of Aeromonas salmonicida, Flavobacterium psychrophilum, and Yersinia ruckeri, three major fish pathogens, by multiplex PCR. Appl Environ Microbiol 68(10):5177–5180
Dong HT, Senapin S, LaFrentz B, Rodkhum C (2016) Virulence assay of rhizoid and non-rhizoid morphotypes of Flavobacterium columnare in red tilapia, Oreochromis spp., fry. J Fish Dis 39:649–655
Dong H, Senapin S, Jeamkunakorn C, Nguyen V, Nguyen N, Rodkhum C, Khunrae P, Rattanarojpong T (2019) Natural occurrence of edwardsiellosis caused by Edwardsiella ictaluri in farmed hybrid red tilapia (Oreochromis sp.) in Southeast Asia. Aquaculture 499:17–23. https://doi.org/10.1016/j.aquaculture.2018.09.007
EI-Jakee J, Elshamy S, Hassan AW, Abdelsalam M, Younis N, El-Hady MA, Eissa AE (2020) Isolation and characterization of Mycoplasmas from some moribund Egyptian fishes. Aquac Int 28(3):901–912. https://doi.org/10.1007/s10499-019-00502-2
Eissa AE, Abou-Okada M, Alkurdi ARM, El Zlitne RA, Prince A, Abdelsalam M, Derwa HIM (2021a) Catastrophic mass mortalities caused by Photobacterium damselae affecting farmed marine fish from Deeba Triangle. Egypt Aquac Res 52:4455–4466
Eissa AE, Attia MA, Elgendy MY, Ismail GA, Sabry NM, Prince A, Mahmoud MA, El-Demerdash GO, Abdelsalam M, Derwa HIM (2021b) Streptococcus, Centrocestus formosanus and Myxobolus tilapiae concurrent infections in farmed Nile tilapia (Oreochromis niloticus). Microb Pathog 158:105084
El Aamri F, Padilla D, Acosta F, Caballero M, Roo J, Bravo J, Vivas J, Real F (2010) First report of Streptococcus iniae in red porgy (Pagrus pagrus, L.). J Fish Dis 33:901–905
El-Adawy MM, Eissa AE, Shaalan M, Ahmed AA, Younis NA, Ismail MM, Abdelsalam M (2020) Green synthesis and physical properties of Gum Arabic-silver nanoparticles and its antibacterial efficacy against fish bacterial pathogens. Aquac Res 52(3):1247–1254. https://doi.org/10.1111/are.14983
Elgendy MY, Kenawy AM, El-Deen AE (2016) Gyrodactylus anguillae and Vibrio vulnificus infections affecting cultured eel, Anguilla anguilla. Comun Sci 7:1–11
Elgendy MY, Soliman WS, Abbas WT, Ibrahim TB, Younes AM, Omara ST (2017) Investigation of some virulence determents in Aeromonas hydrophila strains obtained from different polluted aquatic environments. Jordan J Biol Sci 10:265–272
Elgendy MY, Shaalan M, Abdelsalam M, Eissa AE, El-Adawy MM, Seida AA (2021) Antibacterial activity of silver nanoparticles against antibiotic-resistant Aeromonas veronii infections in Nile tilapia, Oreochromis niloticus (L.), in vitro and in vivo assay. Aquacult Res 53:901–920. https://doi.org/10.1111/are.15632
Elgendy MY, Abdelsalam M, Kenawy A, Ali SE (2022a) Vibriosis outbreaks in farmed Nile tilapia (Oreochromis niloticus) caused by Vibrio mimicus and V. cholerae. Aquac Int. https://doi.org/10.1007/s10499-022-00921-8
Elgendy MY, Abdelsalam M, Mohamed SA, Ali SE (2022b) Molecular characterization, virulence profiling, antibiotic susceptibility, and scanning electron microscopy of Flavobacterium columnare isolates retrieved from Nile tilapia (Oreochromis niloticus). Aquac Int 30:845–862
Elgendy MY, Sherif AH, Kenawy AM (2022c) Abdelsalam M (2022c) Phenotypic and molecular characterization of the causative agents of edwardsiellosis causing Nile tilapia (Oreochromis niloticus) summer mortalities. Microb Pathog 169:105620. https://doi.org/10.1016/j.micpath
Elgendy MY, Moustafa M, Gaafar AY, Borhan T (2015) Impacts of extreme cold-water conditions and some bacterial infections on earthen-pond cultured Nile tilapia, Oreochromis niloticus. RJPBCS6:136–145
El-Hady MA, Ahmed HA (2014) Multiplex PCR for rapid detection of infectious bacterial fish diseases with special reference to Aeromonas hydrophila and Yersinia ruckeri in Egypt. Anim Health Res J 2:284–290
El-Mezayen MM, Rueda-Roa DT, Essa MA, Muller-Karger FE, Elghobashy AE (2018) Water quality observations in the marine aquaculture complex of the Deeba Triangle, Lake Manzala, Egyptian Mediterranean coast. Environ Monit Assess 190:436
El-Son MAM, Nofal MI, Abdel-Latif HMR (2021) Co-infection of Aeromonas hydrophila and Vibrio parahaemolyticus isolated from diseased farmed striped mullet (Mugil cephalus) in Manzala, Egypt – a case report. Aquaculture 530:735738
Essam HM, Abdellrazeq GS, Tayel SI, Torky HA, Fadel AH (2016) Pathogenesis of Photobacterium damselae subsp. piscicida infections in sea bass and sea bream. Microb Pathog 99:41–50
Farzadnia A, Naeemipour M (2020) Molecular techniques for the detection of bacterial zoonotic pathogens in fish and humans. Aquac Int 28:309–320
Fathi M, Dickson C, Dickson M, Leschen W, Baily J, Muir F, Ulrich K, Weidmann M (2017) Identification of tilapia lake virus in Egypt in Nile tilapia affected by ‘summer mortality’ syndrome. Aquaculture 473:430–432
Figueroa C, Bustos P, Torrealba D, Dixon B, Soto C, Conejeros P (2017) Gallar JA (2017) Coinfection takes its toll: sea lice override the protective effects of vaccination against a bacterial pathogen in Atlantic salmon. Sci Rep 7:17817. https://doi.org/10.1038/s41598-017-18180-6
González SF, Krug MJ, Nielsen ME, Santos Y, Call DR (2004) Simultaneous detection of marine fish pathogens by using multiplex PCR and a DNA microarray. J Clin Microbiol 42:1414–1419
Huang M, Chen H, Li C, Liu Y, Gan C, El-Sayed Ahmed MAE, Liu R, Shen C, Zhong R, Tian GB, Huang X, Xia J (2020) Rapid fulminant progression and mortality secondary to Aeromonas dhakensis septicemia with hepatitis B virus infection following the ingestion of snakehead fish in Mainland China: a case report. Foodborne Pathog Dis 17(12):743–749. https://doi.org/10.1089/fpd.2019.2780
Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI (2012) Emerging Aeromonas species infections and their significance in public health. Sci World J 2012:625023. https://doi.org/10.1100/2012/625023
Janda JM, Abbott SL (2007) 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol 45:2761–2764
Kamolvarin N, Tirawatnpong T, Rattanasiwamoke R, Tirawatnpong S, Panpanich T, Hemachudha T (1993) Diagnosis of rabies by polymerase chain reaction with nested primers. J Infect Dis 167:207–210
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1–e1
Kotob MH, Menanteau-Ledouble S, Kumar G, Abdelzaher M, El-Matbouli M (2016) The impact of co-infections on fish: a review. Vet Res 47:98. https://doi.org/10.1186/s13567-016-0383-4
Li J, Zhou L, Woo NYS (2003) Invasion route and pathogenic mechanisms of Vibrio alginolyticus to silver sea bream Sparus sarba. J Aquat Anim Health 15:302–313
Liop P, Bonaterra A, Peñalver J, López MM (2000) Development of a highly sensitive nested-PCR procedure using a single closed tube for detection of Erwinia amylovora in asymptomatic plant material. Appl Environ Microbiol 66:2071–2078
Mahmoud MA, Abdelsalam M, Mahdy OA, El Miniawy HMF, Ahmed ZAM, Osman AH, Mohamed HMH, Khattab AM, Zaki Ewiss MA (2016) Infectious bacterial pathogens, parasites and pathological correlations of sewage pollution as an important threat to farmed fishes in Egypt. Environ Pollut 219:939–948
Mahmoud MA, Attia MM, Abdelsalam M, Abdel-Moneam DA, Zaki Ewiss MA (2021) Ergasilus extensus and bacterial co-infection in flathead grey mullet, Mugil cephalus (Linnaeus, 1758), are associated with pathological changes and immunological gene expression alterations. Aquac Res 52:6143–6151. https://doi.org/10.1111/are.15476
Mata AI, Gibello A, Casamayor A, Blanco MM, Domínguez L, Fernández-Garayzábal JF (2004) Multiplex PCR assay for detection of bacterial pathogens associated with warm-water Streptococcosis in fish. Appl Environ Microbiol 70(5):3183–3187. https://doi.org/10.1128/AEM.70.5.3183-3187.2004
Moustafa M, Eissa AE, Laila AM, Gaafar AY, Abumourad IMK, Elgendy MY (2015) Investigations into the potential causes of mass kills in mari-cultured gilthead sea bream (Sparus aurata) at Northern Egypt. Res J Pharm, Biol Chem Sci 6:466–477
Naas HT, Edarhoby RA, Garbaj AM, Azwai SM, Abolghait SK, Gammoudi FT, Moawad AA, Barbieri I, Eldaghayes IM (2019) Occurrence, characterization, and antibiogram of Staphylococcus aureus in meat, meat products, and some seafood from Libyan retail markets. Veterinary World 12:925–931
Nagamine K, Watanabe K, Ohtsuka K, Hase T, Notomi T (2001) Loop-mediated isothermal amplification reaction using a nondenatured template. Clin Chem 47(9):1742–3. https://doi.org/10.1093/clinchem/47.9.1742
Narayana PS, Varalakshmi D, Pullaiah T, Rao KS (2018) Research methodology in zoology. Scientific Publishers
Nicholson P, Fathi MA, Fischer A, Mohan C, Schieck E, Mishra N, Heinimann A, Frey J, Wieland B, Jores J (2017) Detection of tilapia lake virus in Egyptian fish farms experiencing high mortalities in 2015. J Fish Dis 40:1925–1928
Nicholson P, Mon-on N, Jaemwimol P, Tattiyapong P, Surachetpong W (2020) Coinfection of tilapia lake virus and Aeromonas hydrophila synergistically increased mortality and worsened the disease severity in tilapia (Oreochromis spp.). Aquaculture 520:734746
Okonechnikov K, Golosova O, Fursov M (2012) Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28(8):1166–1167. https://doi.org/10.1093/bioinformatics/bts091
Roberts RJ (2012) Fish pathology. Wiley-Blackwell, W.B. Saunders, Philadelphia
Romalde JL, Lopez-Romalde S, Ravelo C, Magarinos B, Toranzo AE (2004) Development and validation of a PCR-based protocol for the detection of Pseudomonas anguilliseptica. Fish Pathology 39:33–41
Santos Y, Romalde JL, Bandín I, Magariños B, Núñez S, Barja JL, Toranzo AE (1993) Usefulness of the API-20E system for the identification of bacterial fish pathogens. Aquaculture 116:111–120. https://doi.org/10.1016/0044-8486(93)90002-G
Sherif AH, Gouda MY, Naena NA, Ali AH (2020) Alternate weekly exchanges of feeding regime affect the diversity of intestinal microbiota and immune status of Nile tilapia Oreochromis niloticus. Aquac Res 51:4327–4339. https://doi.org/10.1111/are.14778
Sherif AH, Gouda M, Darwish S, Abdelmohsin A (2021a) Prevalence of antibiotic-resistant bacteria in freshwater fish farms. Aquac Res 52:2036–2047. https://doi.org/10.1111/are.15052
Sherif AH, Gouda MY, Al-Sokary ET, Elseify MM (2021b) Lactobacillus plantarum enhances immunity of Nile tilapia Oreochromis niloticus challenged with Edwardsiella tarda. Aquac Res 52:1001–1012. https://doi.org/10.1111/are.14955
Shi Y-H, Chen J, Li C-H, Lu X-J, Zhang D-M, Li H-Y, Zhao Z-X, Li M-Y (2012) Detection of bacterial pathogens in aquaculture samples by DNA microarray analysis. Aquaculture 338–341:29–35. https://doi.org/10.1016/j.aquaculture
Tang YW, Stratton CW (2006) Advanced techniques in diagnostic microbiology. Springer Science, New York, NY. https://doi.org/10.1007/0-387-32892-0
Tom M, Chen N, Segev M, Herut B, Rinkevich B (2004) Quantifying fish metallothionein transcript by real time PCR for its utilization as an environmental biomarker. Mar Pollut Bull 48:705–710
Torres-Corral Y, Santos Y (2021) Development of a real-time PCR assay for detection and quantification of Streptococcus iniae using the lactate permease gene. J Fish Dis 44(1):53–61. https://doi.org/10.1111/jfd.13267
Younes MA, Gaafar YA, Abu-Bryka ZAE, Mohamed AL, Bayoumy ME-S (2019) Genotyping and pathogenicity of Streptococcus iniae strains recovered from cultured Oreochromis niloticus at Kafr El-Shiekh Governorate, Egypt. Egypt J Aquat Biol Fish 23:467–474
Zhou QJ, Wang L, Chen J, Wang RN, Shi YH, Li CH, Zhang YJ (2014) Development and evaluation of a real-time fluorogenic loop-mediated isothermal amplification assay integrated on a microfluidic disc chip (on-chip LAMP) for rapid and simultaneous detection of ten pathogenic bacteria in aquatic animals. J Microbiol Methods 104:26–35
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
This study was conducted in cooperation with all authors.
Corresponding author
Ethics declarations
Ethics approval
The study followed the guidelines and instructions of the Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Cairo University, Egypt.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelsalam, M., Elgendy, M.Y., Elfadadny, M.R. et al. A review of molecular diagnoses of bacterial fish diseases. Aquacult Int 31, 417–434 (2023). https://doi.org/10.1007/s10499-022-00983-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00983-8