Abstract
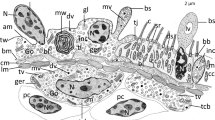
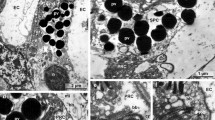
The lens-covered pigment cup ocelli in free-swimming cercariae of Trichobilharzia ocellata were re-examined and the differentiation of the lenticular elements was studied in cercariae still enclosed in sporocysts. Each eye consists of a single rhabdomeric sensory cell, a single cup cell harboring pigment granules and the lens. This lens is developed step by step by the fusion of numerous dark platelets not enclosed by a bordering membrane. The material is presumably proteinaceous. There is no evidence that the lenses are of mitochondrial origin. Besides the pigment cup ocelli, a special type of unpigmented rhabdomeric photoreceptor was discovered. Three unicellular photoreceptors arranged in a three-dimensional configuration exist. These light-sensing organs show a principle of construction similar to that of phaosomous photoreceptors. We hypothesize that both types of photoreceptors are relevant for distinct reactions in the host-finding behaviour of the cercariae. The special type of lensing in the pigmented eyes seems to be restricted to Trichobilharzia species, whereas the phaosomous-like receptors may be more widespread in members of the Schistosomatidae. The obvious absence of any mitochondrial lensing in T. ocellata, and probably all other species of the Digenea, may be correlated with the evolution of endoparasitism in the Trematoda.




Similar content being viewed by others
References
Brooks DR (1989) The phylogeny of the Cercomeria (Platyhelminthes Rhabdocoela) and general evolutionary principles. J Parasitol 75:606–616
Brooks DR, McLennan DA (1993) Parascript. Parasites and the language of evolution. Smithsonian Institution Press, Washington
Cable J, Tinsley RC (1991) The ultrastructure of photoreceptors in Pseudodiplorchis americanus and Neodiplorchis scaphiopodis (Monogenea: Polystomatidae). Int J Parasitol 21:81–90
Dorsey CH, Cousin CE, Fred FA, Stirewalt MA (2002) Ultrastructure of the Schistosoma mansoni cercaria. Micron 33:279–323
Eakin RM (1972) Structure of invertebrate photoreceptors. In: Dartnall HJA (ed) Handbook of sensory physiology, vol VII/I. Springer, Berlin Heidelberg New York, pp 625–684
Ehlers U (1985) Das phylogenetische System der Plathelminthes. Fischer, Stuttgart
Feiler W (1985) Wirtsfindung und Wirtserkennung der Cercariae von Trichobilharzia ocellata. Inauguraldissertation, Fachbereich Biologie, Universität Frankfurt
Feiler W, Haas W (1988) Host-finding in Trichobilharzia occellata cercariae: swimming and attachment to the host. Parasitology 98:493–505
Haas W (2001) Digenea. Host finding. Cercariae. In: Mehlhorn H (ed) Biology, structure, function: encyclopedic reference of parasitology, 2nd edn. Springer, Heidelberg, pp 171–177
Haas W, Haberl B (1997) Host recognition by trematode miracidia and cercariae. In: Fried B, Graczyk TK (eds) Advances in trematode biology. CRC Press, Boca Raton, pp 197–227
Hofsten N von (1918) Anatomie und systematische Stellung von Otoplana intermedia du Plessis. Zool Bidr Uppsala 7:1-74
Horák P, Kolárová L, Adema CM (2002) Biology of the schistosome genus Trichobilharzia. Adv Parasitol 52:155–233
Jamieson BGM (1992) Oligochaeta. In: Harrison FW, Gardiner SF (eds) Microscopic anatomy of invertebrates, vol 7, Annelida. Wiley-Liss, New York, pp 217–322
Kearn GC (1978) Eyes with, and without, pigment shields in the oncomiracidium of the monogenean parasite Diplozoon paradoxum. Z Parasitenkd 157:35–47
Kearn GC (2002) Entobdella soleae—pointers to the future. Int J Parasitol 32:367–372
Kearn GC, Baker NO (1973) Ultrastructure and histochemical observations on the pigmented eyes of the oncomiracidium of Entobdella soleae, a monogenean skin parasite of the common sole, Solea solea. Z Parasitenkd 41:239–254
Littlewood DTJ, Rohde K, Bray RA, Herniou EA (1999) Phylogeny of the Platyhelminthes and the evolution of parasitism. Biol J Linn Soc 68:257–287
Lockyer AE, Olson PD, Littlewood DTJ (2003) Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biol J Linn Soc 78:155–171
Monneron A, Bernhard W (1966) Action des certain enzymes sur des tissues inclus en Epon. J Microsc 5:697–714
Niilonen T (1980) Fine structure of the phaosomous photoreceptor in the larvae of Polydora ligni Webster (Polychaeta: Spionidae). Acta Zool (Stockh) 61:183–190
Pan SC-T (1980) The fine structure of the miracidium of Schistosoma mansoni. J Invert Pathol 36:307–372
Rees FG (1975) Studies on the pigmented and unpigmented photoreceptors of the cercaria of Cryptocotyle lingua (Creplin) from Littorina littorea (L.). Proc R Soc Lond B 188:121–138
Roemer A van de, Haas W (1984) Fine structure of a lens-covered photoreceptor in the cercaria of Trichobilharzia ocellata. Z Parasitenkd 70:391–394
Rohde K (2001) The Aspidogastrea: an archaic group of Platyhelminthes. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor and Francis, London, pp 159–167
Rohde K, Watson NA (1991) Ultrastructure of pigmented photoreceptors of larval Multicotyle purvisi (Trematoda, Aspidogastrea). Parasitol Res 77:485–490
Rohde K, Watson NA, Chisholm LA (1999) Ultrastructure of the eyes of the larva of Neoheterocotyle rhinobatidis (Plathyhelminthes, Monopisthocotyla), and phylogenetic implications. Int J Parasitol 29:511–519
Saladin KS (1982) Schistosoma mansoni: cercarial responses to irradiance changes. J Parasitol 68:120
Short RB, Gagné HT (1975) Fine structure of a possible photoreceptor in cercariae of Schistosoma mansoni. J Parasitol 61:69–74
Snyder SD, Loker ES, Johnston DA, Rollinson D (2001) The Schistosomatidae: advances in phylogenetics and genomics. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor and Francis, London, pp. 194–199
Sopott-Ehlers B (1999a) Submicroscopic anatomy of photoreceptors with mitochondrial lenses in Messoplana floralis (Plathelminthes, Rhabdocoela, "Typhloplanoida"). J Submicrosc Cytol Pathol 31:123–129
Sopott-Ehlers B (1999b) Ultrastructure of ciliary aggregations and unique photoreceptors in Ciliopharyngiella intermedia (Plathelminthes, Neoophora). J Submicrosc Cytol Pathol 31:487–493
Sopott-Ehlers B (2000) First evidence of unpigmented rhabdomeric photoreceptors in a Microstomum species (Plathelminthes, Macrostomorpha, Microstomidae) and ultrastructure of photoreceptor-like ciliary aggregations. Zoomorphology 119:223–229
Sopott-Ehlers B, Kearn GC, Ehlers U (2001a) Evidence for the mitochondrial origin of the eye lenses in embryos of Entobdella soleae (Plathelminthes, Monogenea). Parasitol Res 87:421–427
Sopott-Ehlers B, Salvenmoser W, Reiter D, Rieger R, Ehlers, U (2001b) Photoreceptors in species of the Macrostomida (Plathelminthes): ultrastructural findings and phylogenetic implications. Zoomorphology 121:1-12
Tyler S, Tyler MS (1997) Origin of the epidermis in parasitic platyhelminths. Int J Parasitol 27:715–738
Wright DGS (1974) Responses of cercariae of Trichobilharzia ocellata to white light, and irradiance reduction. Can J Zool 52:575–579
Acknowledgements
The technical assistance of Mrs. P. Freynhagen-Leopold and Mrs. A. Zeise is greatly appreciated. Mr. B. Baumgart kindly helped with the figures. Financial support was provided by the Deutsche Forschungsgemeinschaft (DFG Eh 175/4-1) and the Akademie der Wissenschaften und der Literatur Mainz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sopott-Ehlers, B., Haas, W. & Ehlers, U. Ultrastructure of pigmented and unpigmented photoreceptors in cercariae of Trichobilharzia ocellata (Plathelminthes, Trematoda, Schistosomatidae): evidence for the evolution of parasitism in Neodermata. Parasitol Res 91, 109–116 (2003). https://doi.org/10.1007/s00436-003-0933-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-003-0933-3