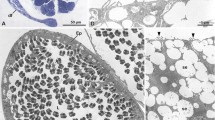
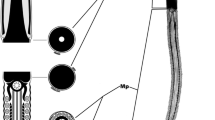
Abstract
Sperm structure of the Xenacoelomorpha (Acoelomorpha plus Xenoturbellida) is updated in the light of new discoveries or new interpretations of existing data. Nemertodermatida and Acoela (Acoelomorpha) have introsperm with certain basic features in common, but all acoels lack acrosomes and usually have two flagella with unusual combinations of microtubules, whereas all nemertodermatids have small, simple acrosomes and a typical 9 + 2 flagellum. Xenoturbellida is currently considered as the sister taxon to Acoelomorpha. Xenoturbella bocki has an aquasperm that has almost nothing in common with the sperm of Acoelomorpha. We argue that the aquasperm ultrastructure of X. bocki has much in common with sperm of hemichordates and to some extent echinoderms, which was previously disputed. Molecular analyses have on the one hand supported a connection with deuterostomes but on the other hand have negated it, suggesting that the closest common ancestor of Xenacoelomorpha is either the Nephrozoa, Deuterostomia or Protostomia. Sperm structure is highly diverse among Xenacoelomorpha, with protostome-like traits in Acoelomorpha and deuterostome-like traits in Xenoturbella. Assuming Xenacoelomorph monophyly and ancestral introsperm in this taxon, however, suggests that the re-expression of the aquasperm form of Xenoturbella, involving some key changes in sperm morphology, is a secondarily derived state that could have occurred through “progenetic spermiogenesis” with the precocious development of round spermatids to maturity.










Similar content being viewed by others
References
Achatz JG, Hooge M, Wallberg A, Jondelius U, Tyler S (2010) Systematic revision of acoels with 9 + 0 sperm ultrastructure (Convolutida) and the influence of sexual conflict on morphology. J Zool Syst Evol Res 48:9–32
Achatz JG, Chiodin M, Salvenmoser W, Tyler S, Martinez M (2013) The Acoela: on their kind and kinships, especially with nemertodermatids and xenoturbellids (Bilateria incertae sedis). Org Divers Evol 13:267–286. https://doi.org/10.1007/s13127-012-0112-4
Baccetti B, Gaino E, Sará M (1986) A sponge with acrosome: Oscularella lobularis. J Ultra Mol Struct Res 94:195–198
Bieler R, Mikkelsen PM, Collins TM, Glover ER, Gonzalez VL, Graf DL, Harper EM, Healy J, Kawanski GY, Sharma PP, Staubach S, Strong EE, Taylor JD, Temkin I, Zardus JD, Clark S, Guzman A, McIntyre E, Sharp P, Giribet G (2014) Investigating the Bivalve Tree of Life—an exemplar-based approach combining molecular and novel morphological characters. Invert Syst 28:32–115
Boone M, Bert W, Claeys M, Houthoofd W, Artois T (2011) Spermatogenesis and the structure of the testes in Nemertodermatida. Zoomorphology 130:273–282
Boyer BC, Smith GW (1982) Sperm morphology and development in two acoel Turbellarians from the Phillippines. Pacific Sci 36:365–380
Brauchle M, Bilican A, Eyer C, Bailly X, Martinez P, Ladurner P, Bruggmann R, Sprecher SG (2018) Xenacoelomorpha survey reveals that all 11 animal homeobox gene classes were present in the first bilaterians. Genome Biol Evol 10(9):2205–2217. https://doi.org/10.1093/gbe/evy170
Buckland-Nicks JA (1995) Ultrastructure of sperm and sperm–egg interaction in Aculifera: implications for molluscan phylogeny. In: Jamieson BGM, Ausio J-L, Justine J (eds) Advances in spermatozoal phylogeny and taxonomy, vol 166. Mémoires du Muséum national d’Histoire Naturelle, Paris, pp 129–153
Buckland-Nicks JA (2006) Fertilization in chitons: morphological clues to phylogeny. Venus 65:51–70
Buckland-Nicks JA (2008) Fertilization biology and the evolution of chitons. Am J Malacol 25:97–111
Buckland-Nicks JA, Chia FS (1986) Formation of the acrosome and basal body during spermiogenesis in a marine snail, Nerita picea (Mollusca: Archaeogastropoda). Mol Reprod Dev 15:13–23
Buckland-Nicks JA, Scheltema A (1995) Was internal fertilization an innovation of early Bilateria? Evidence from sperm structure of a mollusc. Proc R Soc B 261:11–18
Buckland-Nicks JA, Chia FS, Koss R (1990) Spermiogenesis in Polyplacophora, with special reference to acrosome formation (Mollusca). Zoomorphology 109:179–188
Cannon JT, Vellutini BC, Smith J, Ronquist F, Jondelius U, Hejnol A (2016) Xenacoelomorpha is the sister group to Nephrozoa. Nature 530:89–92
Colwin AL, Colwin LH (1963) Role of the gamete membranes in fertilization in Saccoglossus kowalevski (Enteropneusta). 1. The acrosomal region and its changes in early stages of fertilization. J Cell Biol 19:477–500
Colwin AL, Colwin LH (1967) Behavior of the spermatozoon during sperm–blastomere fusion and its significance for fertilization. (Saccoglossus kowalevski: Hemichordata). Z Zellforsch 78:208–220
Dunn CW, Leys SP, Haddock SHD (2015) The hidden biology of sponges and ctenophores. Trends Ecol Evol 30:282–291
Franc J-M (1973) Etude ultrastructurale de la spermatogenèse du Cténaire, Beröe ovata. J Ultrastruct Res 42:255–267
Franzén Å (1955) Comparative morphological investigations into the spermiogenesis among Mollusca. Zool Bidr Upps 30:399–456
Franzén Å (2001) Sperm structure in the enteropneust Schizocardium sp. (Hemichordata, Enteropneusta) and possible phylogenetic implications. Invert Reprod Dev 39:37–43
Friend DS, Fawcett DW (1974) Membrane differentiations in freeze-fractured mammalian sperm. J Cell Biol 63:641–664
Giusti F, Selmi MG (1982) The morphological peculiarities of the typical spermatozoa of Theodoxus fluviatilis (L) (Neritoidea) and their implications for motility. J Ultrastruct Res 78:166–177
Glabe CG, Vacquier VD (1978) Egg surface glycoprotein receptor for sea urchin sperm bindin. Proc Nat Acad Sci USA 75:881–885
Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH (2004) Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature 431:320–325
Haszprunar G (1996) Plathelminthes and Plathelminthomorpha— paraphyletic taxa. J Zool Syst Evol Res 34:41–48. https://doi.org/10.1111/j.1439-0469.1996.tb00808.x
Healy JM (1988) Sperm morphology and its systematic importance in the Gastropoda. Malacol Rev Supp 4:251–266
Healy JM (1996) Molluscan sperm ultrastructure: correlation with taxonomic units within the Gastropoda, Cephalopoda and Bivalvia. In: Taylor J (ed) Origin and evolutionary radiation of the Mollusca. Oxford University Press, Oxford, pp 99–113
Hejnol A, Martindale MQ (2008) Acoel development supports a simple planula-like urbilaterian. Philos Trans R Soc B 363:1493–1501
Hejnol A, Pang K (2016) Xenacoelomorpha’s significance for understanding bilaterian evolution. Curr Opin Genet Dev 39:48–54
Hejnol A. Obst M, Stamatakis A, Ott M, Rouse GW, Edgecombe GD, Martinez P, Baguna J, Bailly X, Jondelius U, Wiens M, Müller WEG, Seaver E, Wheeler WC, Martindale MQ, Giribet G, Dunn CW (2009) Assessing the root of bilaterian animals with scalable phylogenetic methods. Proc R Soc B 276:4261–4270
Hendelberg J (1977) Comparative morphology of turbellarian spermatozoa studied by electron microscopy. Acta Zool 154:149–162
Hendelberg J (1986) The phylogenetic significance of sperm morphology in the Platyhelminthes. Hydrobiologia 133:53–58
Hinsch GW (1974) Comparative ultrastructure of cnidarian sperm. Am Zool 14:457–465
Hodgson AN, Healy JM, Tunnicliffe V (1997) Spermatogenesis and sperm structure of the hydrothermal vent prosobranch gastropod Lepetodrilus fucensis (Lepetodrilidae, Mollusca). Invert Reprod Dev 31:87–97
Jamieson BGM, Rouse GW (1989) The spermatozoa of the Polychaeta (Annelida): an ultrastructural review. Biol Rev 64:93–157
Jondelius U, Ruiz-Trillo I, Baguñà J, Riutort M (2002) The Nemertodermatida are basal bilaterians and not members of the Platyhelminthes. Zool Script 31:201–215
Jondelius U, Wallberg A, Hooge M, Raikova O (2011) How the worm got its pharynx: phylogeny, classification and bayesian assessment of character evolution in Acoela. Syst Biol 60:845–871
Justine J-L (1991) Phylogeny of parasitic Platyhelminthes: a critical study of synapomorphies proposed on the basis of the ultrastructure of spermiogenesis and spermatozoa. Can J Zool 9:1421–1440
Justine J-L (2001) Spermatozoa as phylogenetic characters for the Platyhelminthes. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. CRC Press, Boca Raton, USA, pp 231–238
Kwitny S, Klaus AV, Hunnicutt GR (2010) The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during late steps of spermiogenesis. Biol Reprod 82:669–678
Lundin K, Hendelberg J (1998) Is the sperm type of the Nemertodermatida close to that of the ancestral Platyhelminthes? Hydrobiologia 383:197–205
Lundin K, Sterrer W (2000) The Nemertodermatida. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. CRC Press, pp 24–27
Meyer-Wachsmuth I, Jondelius U (2016) Interrelationships of Nemertodermatida. Org Divers Evol 16:73–84
Obst M, Nakano H, Bourlat SJ, Thorndyke MC, Telford MJ, Nyengaard JR, Funch P (2011) Spermatozoon ultrastructure of Xenoturbella bocki (Westblad 1949). Acta Zool 92:109–115
Onoda M, Djakiew D (1993) A 29000 Mr protein derived from round spermatids regulates Sertoli cell secretion. Mol Cell Endocrinol 93:53–61
Petrov A, Hooge M, Tyler S (2004) Ultrastructure of sperms in Acoela (Acoelomorpha) and its concordance with molecular systematics. Invert Biol 123:183–197. https://doi.org/10.1111/j.1744-7410.2004.tb00154
Philippe H et al (2011) Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature 470:255–260
Phillips DM (1980) Observations on mammalian spermiogenesis using surface replicas. J Ultrastruct Res 72:103–111
Raikova OI, Falleni A, Justine J-L (1997) Spermiogenesis in Paratomella rubra (Platyhelminthes, Acoela): ultrastructural immunocytochemical, cytochemical studies and phylogenetic implications. Acta Zool 78:295–307
Raikova OL, Reuter M, Justine J-L (2001) Contributions to the phylogeny and systematics of the Acoelomorpha. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. CRC Press, Boca Raton, USA, pp 13–23
Rieger RM (1986) Asexual reproduction and the turbellarian archetype. Hydrobiologia 132:35–45
Rouse GW, Wilson NG, Caravajal JI, Vrijenhoek RC (2016) New deep sea species of Xenoturbella and the position of Xenacoelomorpha. Nature 530:94–96
Ruiz-Trillo I, Paps J (2016) Acoelomorpha: earliest branching bilaterians or deuterostomes? Org Divers Evol 16:391–399. https://doi.org/10.1007/s13127-015-0239-1
Ruiz-Trillo I, Riutort M, Littlewood DT, Herniou EA, Baguña J (1999) Acoel flatworms: earliest extant bilaterian Metazoans, not members of Platyhelminthes. Science 283:1919–1923. https://www.ncbi.nlm.nih.gov/pubmed/10082465
Ruiz-Trillo I, Paps J, Loukota M, Ribera C, Jondelius U, Baguñá J, Riutort M (2002) A phylogenetic analysis of myosin heavy chain type II sequences corroborates that Acoela and Nemertodermatida are basal bilaterians. Proc Natl Acad Sci 99:11246–11251. https://www.ncbi.nlm.nih.gov/pubmed/12177440
Summers RG, Hylander BL, Colwin LH, Colwin AL (1975) The functional anatomy of the echinoderm spermatozoan and its interaction with the egg at fertilization. Am Zool 15:523–551
Tyler S, Rieger RM (1975) Uniflagellate spermatozoa in Nemertoderma (Turbellaria) and their phylogenetic significance. Science 188:730–732
Tyler S, Rieger RM (1977) Ultrastructural evidence for the systematic position of the Nemertodermatida (Turbellaria). Acta Zool 154:193–207
Wallberg A, Curini-Galletti M, Ahmadzadeh A, Jondelius U (2007) Dismissal of Acoelomorpha: Acoela and Nemertodermatida are separate early bilaterian clades. Zool Script 36:509–523
Acknowledgements
We are deeply indebted to the work of Seth Tyler, Reinhard Rieger and Jan Hendelberg for the original discovery of the nemertodermatid sperm in the 1970s. Seth Tyler kindly provided J.B-N. with grids that were used for further studies of the sperm of Flagellophora apelti and Andreas Hejnol supplied J.B-N. with fixed specimens of Convolutriloba longifissura and we thank them both very much. In memory of Jan Hendelberg and Reinhard Rieger.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This study was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (#46205) to John Buckland-Nicks.
Conflict of interest
The authors declare that they have no conflicts of interest.
Studies with human participants
This study does not contain studies with human participants performed by any of the authors. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies using animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. All appropriate data created by the authors (i.e., photographs, diagrams) are available in the manuscript.
Rights and permissions
About this article
Cite this article
Buckland-Nicks, J., Lundin, K. & Wallberg, A. The sperm of Xenacoelomorpha revisited: implications for the evolution of early bilaterians. Zoomorphology 138, 13–27 (2019). https://doi.org/10.1007/s00435-018-0425-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-018-0425-8