Abstract
Sperm ultrastructure is frequently employed as a source for phylogenetic inference due to the ease of accessing spermatozoa. Despite being unicellular, sperm cells exhibit a relatively high number of diverse characters and character states. Spermatozoa are subject to strong sexual selection as they are finely tuned for maximizing male reproductive success. Given this strong functional constraint, one might anticipate the emergence of convergent characters in line with similar modes of reproduction. As a result, it might be expected that sperm cells do not possess substantial phylogenetic signal, with functional constraints overshadowing any evolutionary heritage. To test this assumption, we conducted a study on sperm ultrastructure in 11 nemertean species, representing closely related groups and major nemertean lineages. We analyzed these data for their phylogenetic signal within the context of the most recent nemertean phylogenies. Our findings demonstrate that, at all systematic levels, functional constraints imposed by sexual selection, or the mode of reproduction do not supersede the influence of historical constraints on sperm ultrastructure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In terms of comparative morphology sperm cells are among the best studied cell types. They have been examined across nearly all taxonomic levels and within various species, resulting in data available for nearly every high-ranking taxon (Birkhead and Montgomerie 2009; Fitzpatrick et al. 2022). The ultrastructure of spermatozoa is often included in phylogenetic analyses because spermatozoa are relatively easy to obtain and to analyse under an electron microscope, so that data on their ultrastructure are often included into phylogenetic analyses. These data often enhanced resolution of the trees, such as in Jamieson et al. (1999), Dallai (2014), Dallai et al. (2016), Gottardo et al. (2016) for Hexapoda, Tudge (2009) for Crustacea, Giribet and Wheeler (2002) for Bivalvia, Justine (1998, 2014) for Platyhelminthes or Ferraguti and Erséus (1999) for Clitellata. Sperm ultrastructure can also provide additional insights into phylogenetic relationships (Camargo et al. 2016; Campos et al. 2018; Chernyshev et al. 2020; Soley and du Plessis 2020; Quagio-Grassiotto et al. 2020) and help inferring deeper phylogenetic connections (Buckland-Nicks et al. 2019).
Certain sperm characteristics are exclusive to specific groups, suggesting they may be unique traits of larger taxa. For instance, in the case of the Cercomorpha, the largest hexapod subgroup, the presence of nine additional microtubules surrounding the axoneme in their sperm cells supports their monophyly (Dallai et al. 2016). Another example involves the Trepaxonemata within the Platyhelminthes, characterized by biflagellate sperm featuring a central dense core instead of a microtubule duplet (Ehlers 1985). Additionally, some sperm traits are limited to particular groups, such as a midpiece that enwraps the proximal section of the flagellum, a characteristic of vertebrates (van Deurs and Lastein 1973; Fawcett 1975; Jaana and Yamamoto 1981; Asa et al. 1986; Mattei 1988, 1991; Morisawa 1995; Jamieson 2007; du Plessis and Soley 2014). This midpiece consists of an axoneme surrounded by outer dense fibers paralleling the mitochondrial doublets which again are enveloped by a mitochondrial sheath (Gu et al. 2019; Lindemann and Lesich 2016). Notably, this midpiece is absent in other species of the Chordata (Jamieson 1984) and can be considered an evolutionary novelty or autapomorphy of vertebrates.
On the other hand, spermatozoa have evolved significantly to adapt to various modes of reproduction and are subject to sexual selection (Støstad et al. 2018; Lüpold and Pitnick 2018). Owing to these strong functional constraints, they are likely to undergo convergent evolution, especially when considering that sperm cells have limited distinguishing features. Consequently, sperm cells may not contain significant phylogenetic information, as functional constraints shaping their ultrastructure may outweigh historical (phylogenetic) influences. The recent research has indicated that at least at a generic level, functional constraints do not overpower phylogenetic signals (Valchi et al. 2023). In a recent paper Chernyshev et al. (2020) provided some evidence that this also applies to nemertean sperm even at higher taxonomic levels. To investigate whether this holds true at higher taxonomic levels, when additional data are added, we analysed sperm ultrastructure in 11 nemertean species and filled a gap in knowledge by presenting the first sperm ultrastructure in a reptant polystiliferan species, Paradrepanophorus crassus (Quatrefages, 1846). We will use this information to identify unique structures shared by at least two species and will outline hypotheses regarding the phylogenetic significance of these characteristics.
Materials and methods
Animals and light microscopy
The animals studied are collected during the last 15 years at different coastal sites of the North Atlantik and the North Sea as well as in Posidonia meadows of the Mediterranean (Table 1). Most animals were collected during low tide and processed for further studies at the marine biological stations in Concarneau (Brittany, France), Roscoff (Brittany, France) and List (Sylt, Germany) (Alfred-Wegener-Institut Helmholtz-Zentrum für Polar-und Meeresforschung 2023). The Mediterranean species were collected by scientific divers and processed at the Laboratoire Arago in Banyuls-sur Mer (France).
Sperm cells of Paradrepanophorus crassus and Carinoma armandi were analyzed under Nomarsky contrast in an Olympus BX21 light microscope with camera system and documented for metrical analyses
Transmission electron microscopy (TEM)
Parts of male animals that were cast off while removing sediment were fixed in 2.5% glutaraldehyde buffered in 0.05 M phosphate 0.3 M NaCl saline for at least 2 h. L. bilineatus was fixed for 1 week but keeping it the fixative for such a long time negatively influences the quality of preservation. Especially, the nuclei were so densely fixed that we had some problem with infiltration of the resin. After fixation the animals were rinsed four times in the same buffer used during fixation. They were stored in the buffer for up to 6 months prior to postfixation in 1% OsO4 buffered in 0.05 M phosphate 0.3 M NaCl saline for one hour at 4 °C. Subsequently, they were dehydrated in an ascending acetone series followed by propylene oxide and infiltrated with the EMbed 812 (Electron Microscopic Sciences). Polymerization of the resin took 48 h at 60 °C. Series of silver interference-colored sections (70 nm) were cut on diamond knives (DIATOME) in an ultramicrotome (LEICA Ultracut S), mounted on Formvar-covered single slot copper grids and automatically stained with uranyl acetate and lead citrate in a Phoenix autostainer (Nanofilm) and analyzed in a ZEISS EM10CR transmission electron microscope (TEM). Micrographs were taken on DITABIS phosphor imaging plates that were read out in a DITABIS scanner at a resolution of 4000 × 6400 dpi. Contrast and brightness were adjusted with the respective tools in Adobe photoshop.
Scanning electron microscopy (SEM)
Sperm of Tubulanus polymorphus, T. superbus, Lineus longissimus, L. bilineatus and Tetrastemma cerasinum were pipetted into the fixative used for TEM (see above). After 1 h, they were centrifuged at 6000 rpm in a lab centrifuge. The supernatant was replaced by 0.05 M phosphate 0.3 M NaCl saline, the pellet was resuspended and centrifuged again after 10 min at 6000 rpm. This procedure was repeated three times. The last supernatant was replaced by 1% OsO4 buffered in 0.05 M phosphate 0.3 M NaCl saline. After resuspending the sperm cells, they were postfixed for 30 min and dehydrated in an ascending acetone series. During each step within this series, we repeated the procedure of centrifugation, exchanging the ethanol and resuspending the sperm cells. Finally, 100% ethanol was replaced by hexamethyldisalazan (HMDS). One or two drops of resuspended sperm cells were paced on round coverslips. There were mounted on stubs, sputtered with palladium, and analyzed in an TESCAN scanning electron microscope (SEM). Micrographs were digitally recorded.
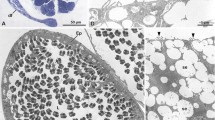
The gonad region of male Tubulanus polymorphus was fixed in alcoholic Bouin fluid (Duboscq Brasil), dehydrated and embedded in Paraffin. We placed 10 µm thick sections that round cover slips, deparaffinized them in xylene, went down the ethanol series and stained them in 7% phosphotungstic acid in 70% ethanol and air dried them after an ascending ethanol series and hexamethyldisalazan (HMDS). The cover slips were mounted on stubs, sputtered with palladium, and studied in TESCAN SEM (Fig. 1A). Micrographs were digitally recorded.
Testes and sperm in Tubulanidae, SEM micrographs. A–C Tubulanus polymorphus, D Tubulanus superbus. A Air dried, deparaffinized 10 µm transverse section of a male. Numerous follicular testes (te) are located below the body wall. B Sperm cells with asymmetric mitochondrion (mi); acrosome (arrowhead) points towards maximal extension of mitochondrion (mitochondrial bulge). C Acrosome (arrowhead) tilted laterally, rectangular to the mitochondrial bulge. bv blood vessel, c cilium, in intestine, lu gut lumen, nc nerve cord
Metrical data
TEM micrographs were used for collecting different metrical data. These data are generally replicates of different sperm cells of one or two individuals (Table 1). Sperm head length was defined by the longitudinal axis of the sperm head. Sperm head width was defined as the widest extension of the head perpendicular to the length measurement.
Line drawings
Every sperm head is represented by a schematic drawing of a midsagittal section generated from consecutive series of parasagittal sections. These schematic reconstructions were generated using Adobe Illustrator. In some cases transverse sections were schematized to provide detailed topographical information on the position of different organelles or dimensional and structural changes along the anterior–posterior sperm axis.
Results
According to this and other studies (Stricker and Folsom 1998; Döhren et al. 2010), but in contrast to Döhren and Bartolomaeus (2006) nemertean spermatozoa consist of a sperm head and a single flagellum. Although the sperm head may sometimes appear to be slightly subdivided, a separate midpiece containing the mitochondria and enwrapping the proximal section of the cilium is not present. In this paper, we therefore choose not to use the term “midpiece”.
The sperm head contains a membrane-bound acrosomal vesicle, a nucleus with highly condensed, electron-dense chromatin, at least one mitochondrion and two centrioles that each consist of nine microtubule triplets. The nucleus may be invaginated to form an anterior nuclear fossa where it faces the acrosome and an implantation fossa where it faces the proximal centriole. In all species examined, the distal centriole gives rise to the 9 × 2 + 2 axoneme of the flagellum. Except for Amphiporus lactifloreus and Paradrepanophorus crassus, a basal plate could not be detected in the nemertean spermatozoa studied (see Döhren et al. 2010 for literature data).
The main sperm axis, the anterior–posterior axis, is defined as longitudinal axis running from the tip of the head (anterior) to the origin of the cilium (posterior). In several cases some organelles are dislocated from this axis. This dislocation is assumed to contain phylogenetic signal (Yurchenko et al. 2021).
Tubulanus polymorphus (Tubulanidae, Palaeonemertea)
Numerous gonads are tightly packed underneath the body wall and the sperm cells are densely packed inside the testes (Fig. 1A). The sperm head of Tubulanus polymorphus is slightly elongated (Figs. 1B, 2G); it measures about 2.4 µm in length and is 2.2 times longer than wide (Table 2). The acrosome is slightly inclined relative to the longitudinal axis of the nucleus; inclination is pointing toward the maximal extension of the asymmetric mitochondrion (Fig. 2G). Since the entire nucleus is also inclined (see below), it obscures the inclination of the acrosome. The acrosome has the shape of an inverted saucer with a thick-walled, electron-dense posterior margin (Fig. 2D). Its diameter is more than two times wider than its anterior–posterior extension (Table 2). The thick-walled posterior margin surrounds a shallow acrosomal pit. Material of moderate electron density fills the space between acrosome and outer cell membrane and forms a small layer in the subacrosomal space (Fig. 2D). This space is topologically defined as space between acrosome and nucleus; it does not include the acrosomal pit, a posterior invagination of the acrosome. The nucleus is slightly ovoid and almost 1.3 times longer than wide (Table 2). At its posterior end an implantation fossa forms a 160 nm deep pit that faces the proximal centriole (Fig. 2F). The head shows a lateral bulge that is caused by the asymmetric, large mitochondrion (Fig. 1C). This organelle is ring shaped (Fig. 2A, B) and located in the posterior half of the sperm head; it extends anteriorly to about half of the total length of the nucleus. The mitochondrion also surrounds the proximal centriole (Fig. 2B). Toward the posterior, the larger portion of the mitochondrion extends beyond the distal centriole (Fig. 2C). Nine small pericentriolar processes (cartwheel processes) radiate from the posterior section of the distal centriole and adhere to the cell membrane (Fig. 2C). A posterior annulus is present.
Tubulanus polymorphus (Tubulanidae). A–F TEM micrographs, G schematic reconstruction. A, B Transverse section of sperm heads at different levels, mitochondrion (mi) surrounds the posterior part of the nucleus (A) and the proximal centriole (B). C Transverse section of the distal centriole with cartwheel extensions adhere to mitochondrial membrane (arrow). D Acrosome, arrowheads mark the electron dense posterior margin. E Longitudinal section along sperm head. Acrosome, nucleus, and posterior centriole are tilted relative to the distal centriole. F An implantation fossa (asterisk) faces the proximal centriole. A posterior annulus (arrow) is present. G Reconstruction of a midsagittal section of the sperm head; position of transverse sections in B and C is indicated, arrows mark posterior annulus. ac acrosome, cwe cartwheel extensions, dc distal centriole, n nucleus, pc proximal centriole
The anterior–posterior axis of the nucleus and the transverse axis of the proximal centriole are tilted by 15°–20° off the anterior–posterior axis of the sperm head which is defined by the main axis of the distal centriole (Fig. 2B). Inclination is always towards the maximum extension of the mitochondrion (Figs. 1B, C, 2E, G). Accordingly, the longitudinal axes of the proximal centriole and the distal centriole are not rectangular but form an obtuse angle of 110°–112° (Fig. 2E, F), similar to T. superbus (Fig. 3G).
Tubulanus superbus (Tubulanidae). A–F TEM micrographs, G schematic reconstruction. A Sagittal section of sperm head, acrosome is not visible in this sectional plane. B Transverse sections of sperm heads, note acrosome with electron dense posterior margin (arrowhead). C Proximal and distal centrioles are not rectangular, asterisk indicates implantation fossa, arrows mark posterior annulus. D Acrosome with electron-dense posterior margin (arrowheads), acrosomal pit (asterisk) and moderate electron dense layer in subacrosomal space (arrow). E Transverse and sagittal section of spermatozoa; mitochondrion surrounds posterior part of nucleus. F Transverse section on the level of the proximal centriole, radial centriolar extensions adhere to mitochondrial membrane (arrow). G Reconstruction of a midsagittal section of the sperm head; position of transverse sections B, F are indicated, arrows mark posterior annulus. ac acrosome, cwe cartwheel extensions, dc distal centriole, mi mitochondrion, n nucleus, pc proximal centriole
Tubulanus superbus (Tubulanidae, Palaeonemertea)
The sperm head of Tubulanus superbus is almost 2.8 µm long and 2.1 times longer than wider. It is slightly larger than that of T. polymorphus (Table 2). Posteriorly it contains a single ring-shaped, slightly asymmetric mitochondrion (Fig. 3A, B, F). Like in T. polymorphus, the acrosome is slightly inclined relative to the longitudinal axis of the nucleus; inclination is pointing toward the maximal extension of the asymmetric mitochondrion (Figs. 1D, 3A). Inclination of the entire nucleus, however, obscures the inclination of the acrosome (Fig. 3G). The acrosome has the shape of an inverted saucer with a thick-walled, electron-dense posterior margin (Fig. 3B, D). The acrosome is more than two times wider than long. The thick-walled posterior margin surrounds an acrosomal pit that is slightly deeper than that of T. polymorphus. Material of moderate electron density fills the space between acrosome and outer cell membrane and form a small layer in the subacrosomal space (Fig. 3D). The nucleus is slightly ovoid and about 1.5 times longer than wide (Table 2). At its posterior end an implantation fossa is present; it is 160 nm deep and faces the proximal centriole (Fig. 3C). The longitudinal axes of the proximal and the distal centriole are not rectangular and form an obtuse angle of 101°–103° instead (Fig. 3C). The mitochondrion is located in the posterior half of the head where it forms a complete ring on the level of the proximal centriole (Fig. 3E, F). Its larger portion extends anteriorly for about a quarter of the entire length of the nucleus and forms an externally visible mitochondrial bulge (Figs. 1D, 3G). Towards the posterior, it extends beyond the distal centriole (Fig. 3F). Nine small pericentriolar processes (cartwheel processes) radiate from the posterior section of the distal centriole (Fig. 3F and adhere to the posterior inner margin of mitochondrion (Fig. 3F). A posterior annulus is present (Fig. 3C).
Like in Tubulanus polymorphus, the anterior–posterior axis of the sperm head slightly tilts towards the longitudinal axis of the distal centriole. Inclination ranges between 11 and 13° but is always rectangular to that side of the sperm cell where the extension of the mitochondrion is maximal (Fig. 3E, G). Accordingly, the position of the acrosome is perpendicular to the mitochondrial bulge (Fig. 1C).
Callinera grandis (Tubulanidae, Palaeonemertea)
The sperm head of Callinera grandis is ovoid and almost 2.2 µm long; it is almost two times longer than wide (Fig. 4A, H; Table 2). The anteriorly located acrosome has the shape of a slightly oval inverted saucer with a thick-walled, electron-dense posterior margin (Fig. 4D, and inset). The acrosome is almost two times wider than long. Its thick-walled posterior margin surrounds an acrosomal pit that is about 70 nm deep. Electron-lucent material forms a small layer in the subacrosomal space, directly underneath the acrosome (Fig. 4D). The nucleus is slightly ovoid and 1.5 times longer than wide (Table 2). At its posterior end an implantation fossa having a depth of about 140 nm faces the anterior edge of the proximal centriole (Fig. 4C). An asymmetric, large mitochondrion forms a complete ring that surrounds the posterior half of the nucleus and the proximal centriole (Fig. 4A, B). The anterior margin of the mitochondrion is oblique to the longitudinal axis of the sperm head. Anteriorly, its larger portion covers the posterior half of the nucleus. Toward the posterior, the larger portion of the mitochondrion extends beyond the distal centriole (Fig. 4B, C, G). The proximal centriole is coaxial to the distal centriole (Fig. 4B, C). Nine small pericentriolar processes (cartwheel processes) radiate from the posterior section of the distal centriole and adhere to the cell membrane (Fig. 4G). A posterior annulus is present. Like in the two Tubulanus species studied, the anterior–posterior axis of the sperm head slightly tilts towards the longitudinal axis of the distal centriole. Inclination ranges between 11° and 13°. It is always oriented to that side of the sperm cell where the extension of the mitochondrion is maximal (Fig. 4H).
Callinera grandis (Tubulanidae). A–G TEM micrographs, H schematic reconstruction. A Sagittal section of sperm head next to mitotic secondary spermatocytes (SPII). Arrows mark acrosomes. B, C Consecutive transverse sections of sperm head (75 nm distance) illustrate anterior and posterior centrioles, asymmetric mitochondrion (mi) and implantation fossa (arrow). D. Transverse section of acrosome (ac). Inset: sagittal section of acrosome; arrowheads mark electron dense posterior margin; scale bar 300 nm. E–G Series of transverse sections as indicated in H shows asymmetric mitochondrion surrounding the proximal centriole (E), but not the distal centriole (F). G Cartwheel extensions adhere to mitochondrial membrane (arrow). H Reconstruction of a midsagittal section of the sperm head; position of micrographs D–G indicated, arrows mark posterior annulus. ac acrosome, cwe cartwheel extensions, dc distal centriole, mi mitochondrion, n nucleus, pc proximal centriole, SPII secondary spermatocyte
Carinoma armandi (Carinomidae, Palaeonemertea)
The sperm head of Carinoma armandi is roughly spherical (Fig. 5A, B) and measures 2.0 µm in length (Table 2). The acrosome has a discoid shape, is 2.6 times wider than long (Table 2) and shows a slightly convex anterior face. The posterior face of the acrosome is concave and tightly parallels the anterior margin of the nucleus. The acrosome is displaced from the sperm’s anterior–posterior axis by 26° and tilted to that side of the sperm cell where the mitochondrion has its maximal extension (Fig. 5G). Due this displacement and its width the anterior tip of the sperm cell is rather stout. The nucleus is an almost perfect sphere measuring about 1.3 µm in diameter. Its posterior margin bears a narrow, 160 nm deep implantation fossa adjacent to the proximal centriole (Fig. 5C). A single, asymmetrical mitochondrion forms a discontinuous ring around the posterior part of the nucleus; the flanks of the mitochondrion do not merge where they meet but form an open ring (Fig. 5D, E). Anterior and posterior margins of the mitochondrion are oblique to the anterior–posterior sperm axis. At its largest anterior–posterior extension, the mitochondrion extends from the posterior half of the nucleus to the posterior margin of the distal centriole. The anterior–posterior extension of the mitochondrion is smallest where the mitochondrial flanks meet (Fig. 5D–F, H). The bigger portion of the mitochondrion is always located on that side of the nucleus to which the acrosome is laterally displaced (Fig. 5G). Proximal and distal centrioles are aligned along the anterior–posterior axis of the sperm head and are perpendicular to each other. The asymmetries in acrosomal position and a mitochondrial bulge can be seen from exterior (Fig. 5A).
Carinoma armandi (Carinomidae). A Light micrograph, Nomarski contrast. B–F TEM micrographs, G, H schematic reconstructions. A Sperm cell. B Transverse and parasagittal sections of sperm cells, C Sagittal section of sperm cell. Note tilted position of acrosome relative to proximal centriole (arrow). D–F. Antero-posterior series of oblique sections showing the topographical relation between mitochondrion, nucleus, and proximal centriole (D, E) and mitochondrion and distal centriole (F). Cartwheel extensions adhere to cell membrane (arrow). G Reconstruction of a midsagittal section of the sperm head; position of electron micrographs D–F is indicated. H Transverse section of the sperm in the level of the proximal centriole, as indicated by the arrow in G. ac acrosome, cwe cartwheel extensions, dc distal centriole, mi mitochondrion, n nucleus, pc proximal centriole
Cephalothrix simula (Cephalotrichidae, Palaeonemertea)
The head of the spermatozoon of Cephalothrix simula is roughly conical and is almost 1.6 times longer than wide (Fig. 6A, F). The acrosome has the shape of a cone with a very broad base which is more than 3.5 times wider than the anterior–posterior length of the acrosome (Table 2). The acrosome sits atop the nucleus but is deflected from the anterior–posterior sperm axis. Inclination is always towards that side which is opposite to the position of the proximal centriole (Fig. 6F). The posterior face of the acrosome is slightly concave; an acrosomal pit is hardly visible (Fig. 6B). A gap of 130 ± 10 nm (n=6) depth separates the acrosome and the nucleus. This gap contains a small band or area of fluffy material of moderate electron density and seems to enfold the posterior circumference of the acrosome (Fig. 6B, F). The nucleus has an almost spherical shape and is merely 1.15 times longer than wide. The nucleus’s posterior pole is invaginated adjacent to the proximal centriole to form an implantation fossa (Fig. 6A, C, D). The mitochondrion forms an incomplete ring that surrounds the posterior one third of the nucleus and the proximal centriole; the mitochondrial flanks do not fuse. The posterior region bulges towards the distal centriole; two mitochondrial lobes can be seen on either side of that centriole (Fig. 6E). The proximal centriole is oblique relative to the transverse axis of the mitochondrion and is laterally dislocated from the distal centriole by half of its diameter (Fig. 6D, F).
Cephalothrix simula (Cephalotrichidae). A–E TEM micrographs, F schematic reconstruction. A Sections of the sperm heads at different levels. B Acrosome and underlying subacrosomal layer of flocculent material of moderate electron density. C Proximal and distal centriole with obliquely oriented axes. D Proximal and distal centriole are not centered, asterisk marks implantation fossa, arrow marks cartwheel extension. E Cartwheel extensions adhere to mitochondrial membrane. F Midsagittal section of the sperm head. ac acrosome, cwe cartwheel extension, dc distal centriole, mi mitochondrion, n nucleus, pc proximal centriole
Lineus longissimus (Lineidae, Heteronemertea)
The sperm cell of Lineus longissimus is bell shaped, since its head is slightly tapering towards the anterior pole; globular mitochondria anterior to the origin of the cilium widen the diameter of the posterior section (Fig. 7C, D). The head is quite slender; it measures 830 ± 20 nm in width and thus is at least 3.8 times longer than wide (Table 2). The acrosome is located anterior to the nucleus and is slightly inclined towards the anterior–posterior sperm axis by up to 22°. The acrosomal vesicle is cone-shaped but consists of two sections, an anterior sphere, and a posterior ring component (Fig. 7A) which both are confluent (Fig. 7B, C). The ring component is 1.3 times wider than the anterior spherical section (Table 1). Due to the ring component, the acrosome shows a shallow posterior invagination of up to 80 nm depth at a diameter of up to 150 nm. This subacrosomal space is confluent with the perinuclear space. The acrosomal vesicle is homogenously filled with electron dense material (Fig. 7B–G). The nucleus is almost cylindrical with a broad and slightly conical tip. It is maximally 2.7 times longer than wide (Table 2). The nucleus is circular in transverse sections (Fig. 7A); the diameter is rather constant for most of its length (Fig. 7B, C). At the anterior margin of the mitochondria, the diameter of the nucleus shrinks drastically to provide space for these organelles. The nucleus terminates with a shallow implantation fossa adjacent to the proximal centriole (Fig. 7B). There are five mitochondria surrounding the narrow, posterior most part of the nucleus as well as the proximal and the distal centriole (Fig. 7C). The mitochondria are almost globular and form a posterior mitochondrial bulge. Each mitochondrion measures 490 ± 50 nm (n = 6) in length and 460 ± 60 nm (n = 10) in diameter (Fig. 7D). Proximal and distal centriole are rectangular, and the former is not offset from the longitudinal axis of the latter. There is a lateral extension to the cartwheel of the distal centriole consisting of two concentric rings that are radially linked to the distal centriole by fibrous material. This cartwheel extension (Fig. 7F) has an of almost 120 nm.
Lineus longissimus (Lineidae). A, B, E–G TEM micrographs, C schematic reconstruction, D SEM micrograph. A Various transverse and semi-transversal sections of sperm cells. B Longitudinal section of sperm head, asterisk marks acrosome. C Reconstruction of a midsagittal section of the sperm head. D Sperm head. E Transversal section, a ring of five mitochondria surrounds the distal centriole. F Semi-transversal section, cartwheel extension of distal centriole with outer concentric ring. G Parasagittal section of acrosomes and semi-transversal section of the mitochondria and posterior part of nucleus. ac acrosome, acrc acrosomal ring component, cwe cartwheel extension, icrc inner concentric ring of cartwheel extension, dc distal centriole, mi mitochondrion, n nucleus, ocrc outer concentric ring of cartwheel extension, pc proximal centriole
Lineus bilineatus (= Siphonenteron bilineatum) (Lineidae, Heteronemertea)
The sperm head of Lineus bilineatus reminds on a flat, stout cone (Fig. 8A, C) with an elliptical diameter. It is 1.8 times longer than wide; on the level of the mitochondria the width increases to maximally 1.9 µm. The acrosome is located anterior to the nucleus. The acrosomal vesicle consists of an anterior conical section and a posterior ring component; both are confluent (Fig. 8D, G). The anterior spherical section is two times longer than wide (Table 2). The acrosomal ring component is wider and measures about 0.5 µm in diameter, but less than 0.1 µm in anterior–posterior extension. Owing to the ring component, the acrosome shows a shallow posterior invagination of maximally 100 nm depth at a maximum diameter of 420 nm. The acrosomal vesicle is homogenously filled with electron dense material (Fig. 8D). The nucleus is roughly conical and attains its widest diameter at the beginning of its posterior half (Fig. 8F, G). The nucleus is maximally 1.25 times longer than wide. At the anterior margin of the mitochondria, the diameter of the nucleus drastically diminishes; the nucleus forms a stout posterior extension that extends into the space between the mitochondria. It ends in a very shallow implantation fossa that lies above the proximal centriole (Fig. 8F). There are five mitochondria surrounding the posterior-most, stout section of the nucleus as well as the proximal and the distal centriole (Fig. 8B). The mitochondria extend slightly posterior to the insertion of the cilium. The mitochondria are 1.36 times longer than wide and each measure 1.02 ± 0.06 µm in length and 750 ± 80 nm (n = 9) in diameter (Fig. 8A, C, D). Proximal and distal centrioles are rectangular and aligned with the anterior–posterior axis of the sperm cell (Fig. 8G). A cartwheel is present; its extensions adhere to the cell membrane (Fig. 8E).
Lineus bilineatus (Lineidae). A, B, D–F. TEM micrographs, C SEM micrograph, G schematic reconstruction. A Sagittal section of the sperm cell; arrows mark acrosomes. B Five mitochondria surround the posterior section of the nucleus and the distal centriole (pc). C Sperm head; acrosome is aligned with the longitudinal sperm axis. D Acrosome (ac) with acrosomal ring component (arrowheads). E Cartwheel extensions of distal centriole pass mitochondria. F Cartwheel extensions connected by an inner and an outer ring component. G Midsagittal section of the sperm head; arrow marks cartwheel extension. ac acrosome, acrc acrosomal ring component, c cilium, dc distal centriole, icrc inner concentric ring of cartwheel extension, mi mitochondrion, n nucleus, ocrc outer concentric ring of cartwheel extension, pc proximal centriole
Paradrepanohorus crassus (Drepanophoridae, Hoplonemertea)
The sperm head of the polystiliferan species Paradrepanophorus crassus is elongated with a rounded, slightly inclined tip (Fig. 9H). Its posterior one third bulges slightly, is flat and houses a single large mitochondrion (Fig. 9A, B, I). The sperm head is roughly 12 µm long and it is measures up to 0.45 µm in width its middle section, but up to 0.86 µm in width in its posterior section (Table 2). Relative to the middle section the sperm is 27 times longer than wide. The acrosomal vesicle is situated apically, but slightly tilted towards the cell’s longitudinal axis (Fig. 9D). It has the shape of a bell with a stronger involute posterior margin and is horseshoe-shaped in longitudinal sections. It is almost long as wide and contains homogeneously stained material; the acrosomal pit is a deep invagination of the acrosome (Fig. 9C, D; Table 2). The acrosome seems to contact the anterior margin of the nucleus, which shows a conspicuous cup-shaped invagination of 60 ± 5 nm (n = 6) depth (Fig. 9C). The nucleus is long, roughly cylindrical but circular in cross-section (Fig. 9I). It has a length of about 11 µm and a diameter of 0.4 µm (Table 2) that diminishes posteriorly into a rod with a diameter of 0.2 µm ± 30 nm (n = 21). This rod runs parallel to the mitochondrion towards the proximal centriole, where is widens again to form a cup-shaped implantation fossa (Fig. 9F, G). The mitochondrion is 5.13 ± 0.5 µm (n = 6) long and bean-shaped in cross-section (Fig. 9A). Towards its posterior margin it becomes very flat, surrounds three quarters of the proximal centriole, and ends on the level of the distal centriole (Fig. 9E, I). Both centrioles are rectangular and aligned with the same axis, but slightly offset from the anterior–posterior sperm axis (Fig. 9G). Some electron-dense material fills the space between centrioles (Fig. 9E). A cartwheel is present but shows no ultrastructural peculiarities. The central pair of axonemal microtubules adheres to a basal plate (Fig. 9G).
Paradrepanophorus crassus (Drepanophoridae). A–G TEM, H light microscopy, I schematic reconstructions. A, B Transverse sections of sperm cells. A single mitochondrion runs parallel to the distal section of the nucleus and almost surrounds the proximal centriole (arrows in A). C, D Acrosome is ring-shaped in cross section (C) and cup-shaped in sagittal section (D). Note subacrosomal invagination of the nucleus (arrow). E–G Parasagittal sections of centrioles; arrows mark distal end of mitochondrion, asterisks in E, F intercentriolar electron dense material, and arrow head in G the implantation fossa. H Sperm cells under Nomarski contrast. I Schematic longitudinal section of sperm cell with transverse sections as indicated. Note the differing scale bar in schemes of midsagittal and transverse sections. ac acrosome, bp basal plate, dc distal centriole, mi mitochondrion, n nucleus, pc proximal centriole
Tetrastemma cerasinum (Tetrastemmatidae, Hoplonemertea)
The sperm head of the monostiliferan Tetrastemma cerasinum is elongated concial with five to six ridges and a slightly inclined tip (Fig. 10A). Its posterior half bulges slightly towards this inclination and houses a single large mitochondrion (Fig. 10H). Due to the ridges, transverse sections of the sperm cells are polygonal. The diameter ranges between 0.8 and 1 µm, so that the sperm head is 7–8.7 times longer than wide (Fig. 10A, G; Table 2). The acrosomal vesicle is situated apically and is inclined to the cell’s longitudinal axis by 12°. The acrosome is elongated bell-shaped, contains homogeneously stained material and is nearly twice as long as wide (Fig. 10B). The inner space of the bell, the acrosomal pit, is approximately 310 nm deep and 70 nm wide (Table 1). The acrosome is underlain by a ring-shaped electron-dense subacrosomal condensation that is not membrane bound (Fig. 10C, D). This structure has a diameter of 246 ± 7 nm (n = 7) and is 95 ± 12 nm (n = 7) thick. A small subacrosomal space separates this condensation and the nucleus. In the specimen studied the perinuclear cisterna was artificially enlarged, so that the exact distance between condensation and nucleus could not be determined. Underneath this condensation the nucleus shows an invagination, the anterior fossa. The anterior fossa forms a shallow cup that is slightly oblique relative to the anterior–posterior sperm axis and is less than 60 nm deep (Fig. 10B). The nucleus is around 6 µm long and bears 5–7 longitudinal ridges. These ridges cause a grooved external structure of the sperm cell. The ridges are lesser prominent where the mitochondrion parallels the nucleus. Directly anterior to the mitochondria the diameter of the nucleus is largest and measures about 700 nm in diameter (Table 2). Towards the anterior tip the nucleus’ diameter decreases to 330 ± 44 nm (n = 7). Posteriorly, the nucleus forms an almost spherical implantation fossa that is dislocated from the central anterior–posterior axis of the nucleus and houses most of the proximal centriole (Fig. 10E, F). On the level of the anterior margin of the nuclear fossa a single mitochondrion surrounds the nucleus almost completely (Fig. 10H). Two branches of the mitochondrion extend anteriorly; each of them is almost crescent in transverse section (Fig. 10G, H). Since each branch parallels the nucleus for more than 2 µm, the two branches may lead to the impression that the posterior half of the nucleus is surrounded by two mitochondria. Proximal and distal centrioles are perpendicular but do not share the same axis. The distal centriole is dislocated from the anterior–posterior axis of the sperm (Fig. 10E, F). Some electron-dense material is deposited between centrioles (Fig. 10E). A cartwheel is present but shows no ultrastructural peculiarities.
Tetrastemma cerasinum (Tetrastemmatidae). A SEM micrograph, B–G TEM micrographs, H schematic reconstructions. A Sperm cell with characteristic external ridges (arrow). B Midsagittal section of the sperm cell, rectangular to proximal centriole. Arrow marks anterior nuclear fossa. C, D Acrosome (ac) and subacrosomal condensation (sac). E Proximal and distal centrioles are not aligned with the sperm’s anterior–posterior axis. Note electron-dense material connecting both centrioles. F The distal centriole is not centered relative to the proximal centriole, arrow marks distal margin of mitochondrion. G Various transverse sections of sperm cells. The mitochondrion incompletely surrounds the basal section of the nucleus. H Midsagittal section and representative transverse sections of the sperm head, reconstructed from different series of sections; arrow marks the subacrosomal condensation. ac acrosome, dc distal centriole, mi mitochondrion, n nucleus, pc posterior centriole, sac subacrosomal condensation
Nemertopsis flavida (Emplectonematidae, Hoplonemertea)
The sperm head of the monostiliferan Nemertopsis flavida is more or less egg-shaped with a broad posterior and a small anterior end. The sperm head measures up to 3.32 µm in anterior–posterior extension and up to 1.7 µm maximal width in its posterior section (Fig. 11A, D). The acrosomal vesicle is situated apically and reminds on a flat bell as its diameter is almost 1.5 times wider than its anterior–posterior extension (Table 2). It consists of a small cap-like anterior section and a bulbous ring-like posterior section. The content of both sections is confluent and contains homogeneously stained material (Fig. 11B, C). The posterior invagination 1.3 times deeper than wide (Table 2). A core of moderate electron-density lies inside this invagination and measures 180 ± 4 nm (n = 5) in length and 60 nm (n = 5) in diameter. This core extends beyond the posterior margin of the acrosome into small subacrosomal space and is not surrounded by a membrane (Fig. 11B). The anterior fossa of the nucleus is slightly cup-shaped and 130 nm deep (Fig. 11B). The nucleus has the general shape of a bulged cylinder and is 1.6 times longer than its widest diameter. Its maximum diameter measures 1.1 µm, but its width decreases towards the anterior and the posterior tips (Fig. 11D). Posteriorly, the nucleus is invaginated to form a 160 nm deep, cup-shaped implantation fossa that surrounds the anterior half of the proximal centriole. A single mitochondrion surrounds the posterior section of the nucleus and resembles an egg cup (Fig. 11E). Posteriorly the mitochondrion extends to the posterior margin of the distal centriole (Fig. 11F, G). Proximal and distal centrioles are almost perpendicular, and some electron-dense material is deposited between centrioles (Fig. 11A). The distal centriole bears an anchoring device that consists of conspicuous bulbous endings of the cartwheel. These are rather connected to the cell membrane than to the mitochondrion (Fig. 11F, G).
Nemertopsis flavida (Emplectonematidae). A–C, E–G. TEM micrographs, D schematic reconstruction. A Transverse, oblique and parasagittal sections of sperm cells. B Longitudinal section of anterior portion of sperm cell showing acrosome, acrosomal rod and the anterior nuclear fossa. C Arrows mark cartwheel extensions of the distal centriole. D Midsagittal section of the sperm head, reconstructed from series of sections; arrow marks cartwheel extensions. E A single mitochondrion encircles the basal part of the nucleus. F, G Cartwheel extensions consist of conspicuous bulbous endings. ac acrosome, ar acrosomal rod, dc distal centriole, mi mitochondrion, n nucleus, pc proximal centriole
Amphiporus lactifloreus (Amphiporidae, Hoplonemertea)
The sperm head of the monostiliferan species Amphiporus lactifloreus is elongated with a slightly acute tip. The posterior one fifth bulges slightly and houses a single circular mitochondrion that branches out towards the anterior (Fig. 12A, G). The sperm head is elongated; it measures more than 12 µm in length but less than 0.6 µm in width and thus is almost 22 times longer than wide (Table 2). The conical, long acrosome is coaxial to the anterior–posterior axis of the sperm cell, measures up to 800 nm in length and is at least 4 times longer than wide. The acrosomal pit is deeply invaginated being almost ten times deeper than wide (Table 2). The pit contains some material lesser electron-dense than the content of the acrosome. This material is not surrounded by a membrane. It forms a rod inside the acrosomal pit, broadens posteriorly and seems to plug the acrosomal pit (Fig. 12C). The nucleus has a length of more than 10 µm and tapers towards the acrosome. The nucleus bears generally 4 ridges that are maximally 72 nm high and give the nucleus a tetra-radiate cross-section (Fig. 12A, B). These ridges twist slightly during its anterior–posterior course and cause a grooved surface of the sperm cell. The maximum diameter of the nucleus increases during its course to the mitochondria from less than 200 nm directly underneath the acrosome up to 500 nm anterior to the mitochondrion. Here, the diameter of the nucleus decreases rapidly, and the ridges disappear. The nucleus is rod shaped now with a circular outline and a diameter of 176 ± 8 nm (n = 8) (Fig. 12G). The nucleus terminates next to the proximal centriole by forming an implantation fossa that has the shape of an ‘‘M’’ in sagittal section (Fig. 12E, G). A single 1.8 ± 0.17 µm (n = 8) long mitochondrion forms a collar around the nuclear rod and the centrioles. Next to the proximal centriole it forms a complete ring (Fig. 12B, G). Towards the anterior this ring runs out into four branches that follow the course of the nuclear ridges for less than one micrometre. The proximal centriole is surrounded by 9 electron-dense dots that are aligned with its microtubule-triplets (Fig. 12E, F). The centrioles are largely coaxial and aligned along the longitudinal axis of the head.
Amphiporus lactifloreus (Amphiporidae) A–F TEM, G schematic reconstructions. A, B Transverse sections of sperm cells. Nucleus tapers towards the tip (arrow) and has nuclear ridges, the mitochondrion encircles the nuclear rod. A Wall of testis, B center of testis, arrows mark transversely sectioned acrosomes. C Longitudinal section of acrosome, asterisk marks subacrosomal space. D–F Centrioles and pericentriolar structures. D Longitudinal section of proximal and distal centriole, white asterisk marks electron dense dot, arrows mark distal end of mitochondrion. E and F Transverse section of proximal centriole, midsagittal section of distal centriole. Arrows mark pericentriolar electron-dense dots, arrowhead implantation fossa., mitochondrion partially encircles distal centriole (E). G Schematic midsagittal section of sperm cell with transverse sections as indicated. Scale bar applies to all schemes. ac acrosome, dc distal centriole, ecm extracellular matrix, mc muscle cell, mi mitochondrion, n nucleus, pc proximal centriole
Discussion
Since sperm cells universally serve the same purpose in all sexually reproducing species and, at least in theory, should be subject to strong functional constraints, they remain among the most variable cell types within multicellular animals (Fitzpatrick et al. 2022). Some of this morphological variability can be attributed to the mode of fertilization and the environmental conditions in which sperm cells operate (Franzén 1956; Jamieson and Rouse 1989; Rouse 2005; Kahrl et al. 2021). However, there are instances where larger animal groups can be distinguished by their sperm ultrastructure (Justine 1998, 2014; Ferraguti and Erséus 1999; Giribet and Wheeler 2002; Tudge 2009; Dallai et al. 2016; Buckland-Nicks et al. 2019; Quagio-Grassiotto et al. 2020). This implies that sperm cells may indeed carry some phylogenetic signal that is not outbalanced by functional constraints. In this section, we will explore and validate potential phylogenetic information within sperm cells, specifically focusing on their role in supporting the monophyly of certain nemertean taxa.
Recent molecular studies have provided a solid foundation for consistent hypotheses regarding nemertean phylogeny, as summarized in Fig. 13. These hypotheses will serve as framework for assessing the potential phylogenetic signal present in sperm ultrastructure. Our findings largely align with previous assumptions regarding potential autapomorphies and underscore the significance of sperm ultrastructure in deciphering nemertean phylogeny (Döhren et al. 2010; Chernyshev et al. 2020; Yurchenko et al. 2021).
Current status of nemertean phylogeny, modified from Kajihara et al. (2022a, b) (Piliodiophora), Chernyshev et al. (2021a, b, 2022) (Palaeonemertea), Chernyshev and Polyakova (2019), Kajihara (2021) (Hoplonemertea). Tetrastemmidae are paraphyletic within Amphiporina (Chernyshev et al. 2021a, b). Names of higher taxa according to Chernyshev (2021). Potential autapomorphies in sperm ultrastructure: 1 single mitochondrion; 2 inclined acrosome; 3 five mitochondria; 4 acrosomal ring component; 5 anterior nuclear fossa; 6 elongated hollow cone shaped acrosome. Characters 3 and 4 confirm Döhren et al. (2010) and Chernyshev et al. (2020), character 2 confirms Yurchenko et al (2021), characters 1, 5 and 6 confirm Döhren et al. (2010)
Palaeonemertea
Callinera grandis as well as Callinera sp. from the Sea of Japan share an acrosome that is offset of the longitudinal sperm axis (Yurchenko et al. 2021; this study). A similar inclination is described in this paper in Carinoma armandi, Cephalothrix simula, Lineus longissimus and Tetrastemma cerasinum. It is also described form the paleonemerteans Carinoma mutabilis Griffin, 1898, Cephalothrix rufifrons (Johnston, 1837), Cephalothrix (= Procephalothrix) filiformis (Johnston, 1828), Cephalothrix (= Procephalothrix) oestrymnicus Junoy and Gibson, 1991, the pilidiophoran Hubrechtella juliae Chernyshev, 2003, and the hoplonemerteans Zygonemertes virscens (Verrill, 1879), Malacobdella grossa (Müller, 1776) and Antarctonemertes phyllospadicola (Stricker, 1985) (Afzelius 1971; Stricker and Cavey 1986; Jespersen 1994; Döhren et al. 2010). A slight inclination of the acrosome is also seen in sperm cells in Tubulanus polymorphus and T. superbus (this paper) as well as in Tubulanus sexlineatus (Griffin, 1898) and T. polymorphus from the Pacific East Coast (T. polymorphus: Stricker and Folsom 1998: Figs. 5C, 6C; Döhren et al. 2010: Fig. A, B; T. sexlineatus: Stricker and Folsom 1998: Fig. 6C). This deviation from an apical position of the acrosome has been assumed to be an autapomorphy of palaeonemerteans (Yurchenko et al. 2021). An acrosome that is aligned with the longitudinal axis should then be an autapomorphy for Nemertea. Since sperm cells of Carinina ochracea Sundberg, Chernyshev, Kajihara, Kånneby and Stand, 2009 (as Tubulanus linearis (McIntosh, 1874) in Döhren et al. (2010), see Döhren (2016)), however, do not show such an inclined acrosome, the coaxial position of its acrosome should be secondary. Given that inclination of the acrosome is also observed in some heteronemertean and hoplonemertean species, hypothesizing a secondarily gained coaxial position is presently equally parsimonious as assuming repeated evolution of an altered, inclined position of the acrosome. Except for C. ochracea, however, an inclined acrosome is found in all sperm cells of palaeonemertean species studied thus far, so that this character could hint at a basal position of Carininidae within Palaeonemertea and characterize the remaining palaeonemerteans as their sister group, other than shown in Fig. 13. Chernyshev et al. (2021a), however, discuss a single ventral eye in the larvae of carinomids and carininids as hint for their sister group relationship, but our data show clear ultrastructural differences between the eyes of both (Döhren 2009; unpublished data).
Tubulaniformes
A cluster consisting of the three genera Parahubrechtia, Tubulanus, and Callinera was discovered by Chernyshev and Polyakova (2019) as one of the results of a large molecular analysis of nemerteans including several Deep-Sea species (see also Chernyshev et al. 2022). In this analysis these three genera form a large monophyletic palaeonemertean subgroup, the Tubulanidae (= Tubulaniformes (Chernyshev 2021)), that consist of continental shelf species as well as intertidal, abyssal and hadal species. Morphological characters supporting this group are presently lacking. Recently, Yurchenko et al. (2021) published first ultrastructural data on the sperm cells of a Parahubrechtia species from the Peter the Great Bay (Sea of Japan). This species shows an electron-dense condensation in the acrosomal membrane restricted to the posterior section that surrounds the shallow acrosomal pit (Yurchenko et al. 2021: Fig. 8F). This unusual condensation is also described and visualized for an undescribed Callinera species form the Peter the Great Bay (Sea of Japan) (Yurchenko et al. 2021: Fig. 7E). We found such a condensation also in the sperm cells of Callinera grandis, Tubulanus polymorphus from France and T. superbus described in this paper, as well as in Tubulanus sexlineatus and T. polymorphus from the Pacific East Coast of the USA which according to Krämer (2017) is another species than T. polymorphus from France (Stricker and Folsom 1998; Fig. 5C, G; Döhren et al.2010; Fig. 5B). Such an electron dense condensation in the peripheral posterior membrane of the acrosome is thus far unknown from any other nemertean species and is identical in terms of position and structure in the members of the three genera. We assume that this condensation evolved in the common stem lineage of these three genera (Fig. 13) and offer morphological support for the clade identified by Chernyshev and Polyakova (2019). Since such a condensation has thus far not been described in other nemertean sperm, it could be regarded as autapomorphy of the Tubulaniformes.
Pilidiophora
Pilidiophora was identified a monophyletic by Thollesson and Norenburg (2003); it consists of Hubrechtiiformes and Heteronemertea as highest-ranking sister groups (Chernyshev 2021). All recent molecular analyses support this sister group relationship (Andrade et al. 2012, 2014). Morphologically, this taxon is supported by a special larva, the pilidium, in which the juvenile develops from imaginal plates in the inner of the larva. Such a larva is missing outside the Pilidiophora, so that it must have evolved in the common stem lineage of Hubrechtiiformes and Heteronemertea. In a previous study we provided evidence that several mitochondria per sperm cell are a potential autapomorphy of the Heteronemertea, or potentially of Pilidiophora, if our interpretation of light microscopical images in Franzén (1956) was confirmed (Döhren et al. 2010). This has recently been done in a paper of Chernyshev et al. (2020) who studied the sperm ultrastructure in Hubrechtella juliae and a basally branching heteronemertean, the valenciid Sonnenemertes cantelli Chernyshev, Abukawa and Kajihara, 2015. Sperm cells of all pilidiophoran species studied thus far possess at least three mitochondria, the majority of them five mitochondria (Table 3). Outside the Pilidiophora a single mitochondrion is found, except for the hoplonemerteans Malacobdella grossa and Nectonemertes mirabilis Verrill, 1892, and an undetermined Callinera species (Afzelius 1971; Stricker and Folsom 1998; Yurchenko et al. 2021). Due to their phylogenetic position, it is likely that an increased number of mitochondria evolved independently in both, so that a single mitochondrion represents the primary condition in Nemertea (Döhren et al. 2010; Yurchenko et al. 2021). In several non-pilidiophoran nemertean species spermiogenesis was studied in, several mitochondria fuse to form a single one (Stricker and Folsom 1998; Yurchenko and Chernyshev 2022). These observations allow inferring that several mitochondria evolve by a truncated process of mitochondrial fusion (Döhren et al. 2010; Yurchenko et al. 2021). This scenario of a progenetic spermatogenesis is profoundly discussed in Yurchenko et al. (2021) and explains why several mitochondria most parsimoniously represent a derived condition. One thus would expect to find interspecifically variable numbers of mitochondria whenever more than a single mitochondrion is present. This is actually the case in Callinera sp. (Yurchenko et al. 2021) but can hardly be found in pilidiophoran species. Instead, the number of mitochondria is largely species-specific in Pilidiophora and ranges between three and five (Table 3). Since the mitochondria are located in the posterior region of the sperm cell where they surround the stalk-like posterior section of the nucleus, they cause a bulged posterior section that is expected to influence the swimming behavior. We assume that the number of mitochondria is biomechanically constraint and thus less variable than assumed. The primary number of mitochondria in Pilidiophora presently seems to be five, since the basalmost branching representatives possess 5 mitochondria as many ingroup species also do (Table 3; Fig. 13).
Heteronemertea
Several attempts have been made and enormous efforts were put into solving the ingroup relationships of Heteronemertea (Schwartz and Norenburg 2001; Schwartz 2009; Kvist et al. 2015; Gonzalez-Cueto et al. 2015; Chernyshev et al. 2018; Kajihara et al. 2022a, b). In addition to a few genera and some monotypic or oligotypic families, Valenciniidae and Lineidae are the species richest groups within Heteronemertea (Kajihara et al. 2022a). Roughly 76 genera of the Lineidae are monotypic or rarely oligotypic. The majority of the lineid species is traditionally classified into one of the three genera, Micrura, Cerebratulus, and Lineus. There is strong evidence that none of these is monophyletic (Sundberg and Saur 1998; Kvist et al 2014, 2015; Chernyshev and Polyakova 2022; Chernyshev et al. 2018; Kajihara et al.2022b; Sagorny et al. 2022). In several heteronemertean species, a posterior acrosomal ring component has been described (Table 3), a structure that is missing in the outgroup (Döhren et al. 2010; Chernyshev et al. 2020) and in the ingroup member Sonnenemertes cantelli (Valenciniidae) (Chernyshev et al. 2020). Such a posterior ring component is part of the acrosome surrounded by the acrosomal membrane. This is a strong difference to the subacrosomal condensation, described in this paper for the monostiliferan hoplonemertean Tetrastemma cerasinum, since this structure is not a membrane bound organelle, independent of and posterior to the acrosome. A posterior ring component is presently unique to those lineid species that were analyzed for sperm ultrastructure. Our data on the sperm cells of Lineus longissimus and L. bilineatus thus corroborate the hypothesis of von Döhren et al. (2010) and Chernyshev et al. (2020) according to which the posterior ring component of the acrosome is an autapomorphy of Lineidae (Fig. 13).
Hoplonemertea
Hoplonemertea comprises Polystilifera and Monostilifera as sister groups (Sagorny et al. 2022). Stricker and Folsom (1998) provided first data on the sperm ultrastructure of a polystiliferan, the pelagic Nectonemertes mirabilis. Their sperm cells possess several mitochondria and a cilium equipped with a rootlet. The quality of fixation, however, precluded further details. Paradrepanophorus crassus is a member of the presumably paraphyletic reptant polystiliferans (Chernyshev and Polyakova 2018; Sagorny et al. 2022). It shows characters that are also found among monostiliferan sperm cells, like an anterior nuclear fossa (except for Emplectonema gracile (Johnston, 1837) (Döhren et al. 2010), but present in Emplectonema neesi (Örsted, 1843) (Whitfield 1972: Fig. 2). Such an anterior nuclear fossa has thus far not been described outside Hoplonemerteans (Döhren et al. 2010). An elongated mitochondrion that causes a stalk-like posterior section of the nucleus is described for the hoplonemertean Amphiporus lactifloreus, Amphiporus imparispinosus Griffin, 1898, Paranemertes peregrina Coe, 1901, Emplectonema gracile, E. neesi, Gononemertes australiensis Gibson, 1974, Gononemertes parasita Bergendal, 1900 but is absent in Tetrastemma cerasinum, Amphiporus cruentatus Verrill, 1879 and Nemertopsis flavida (Whitfield 1972; Egan and Anderson 1979; Turbeville and Ruppert 1985; Franzén and Sensenbaugh 1988; Döhren et al. 2010; this paper). This character, however, is also found in Lineus viridis (Müller, 1774) but is certainly not homologous, since this species clearly belongs to the heteronemertean radiation as it possesses three mitochondria (Döhren and Bartolomaeus 2006). A stalk-like posterior section of the nucleus may be functionally constraint by the slenderness of the sperm cell along with elongated, anteriorly projecting mitochondria.
Amphiporidae
Amphiporidae is a group of amphiporine hoplonemerteans that were recovered as monophyletic in a molecular-phylogenetic analysis by Chernyshev and Polyakova (2019). This group includes the type species of Amphiporus, Amphiporus lactifloreus, further Amphiporus species, Paranemertes peregrina and representatives of further genera like Zygonemertes virescens (Verrill, 1879). Sperm ultrastructure in A. imparispinosus and P. peregrina shows an elongated, hollow cone-shaped acrosome that is at least 6 times longer than wide (cluster 1 in Döhren et al. 2010: Fig. 11B). This is confirmed for A. lactifloreus in this paper. If such elongated, hollow cone-shaped acrosome represented an autapomorphy of this group, Emplectonema should also belong to Amphiporidae. The species of this group also share elongated mitochondria with finger-like extensions to the anterior, and a minimum of four nuclear grooves. The emplectonematid Nemertopsis flavida and the amphiporids Zygonemertes virescens and Amphiporus cruentatus, however, possess short or slightly elongated sperm cells and lack such an elongated acrosome as well as finger-like mitochondrial extensions. This could either hint at a functional correlation between sperm length and acrosome or at hidden monophyletic groups among Amphiporina. The former, however, seems unlikely, since elongated sperm outside monostiliferan nemerteans do not correlate with elongated acrosomes (Döhren und Bartolomaeus 2006 for Lineus viridis, this study for Paradrepanophorus crassus). The latter may receive support by molecular evidence showing that Amphiporus cruentatus belongs to the Oerstediina (Chernyshev and Polyakova 2002). However, further molecular and morphological data are needed to confirm this hypothesis.
Conclusions
In summary, our study confirms previous interpretations of the evolution of substructures in nemertean sperm cells (Stricker and Folsom 1998; Döhren et al. 2010; Chernyshev et al. 2020; Yurchenko et al. 2021). It also highlights the potential contribution of sperm ultrastructure as a valuable source of characters for phylogenetic inferences. Furthermore, our findings support the notion that historical constraints have influenced sperm ultrastructure, although functional constraints may modulate these characteristics. Functional demands can lead to convergent evolution of certain traits, such as the stalk-like posterior nucleus section in Lineus species and Amphiporidae, driven by the tradeoff between slender sperm size and mitochondrial placement. Conversely, other characters, such as the electron-dense reinforcement of the posterior acrosomal section in tubulaniform nemerteans, an increase in mitochondrial numbers in Pilidiophora, and the acrosomal ring component, appear to be less influenced by functional constraints and are likely inherited features shaped by historical constraints that support the monophyly of Pilidiophora and Tubulaniformes.
Data Availability
All data the study has been based on are available at the corresponding author upon request.
References
Afzelius B (1971) The spermatozoon of the nemertine Malacobdella grossa. J Submicrosc Cytol 3:181–192
Alfred-Wegener-Institut Helmholtz-Zentrum für Polar-und Meeresforschung (2023) Marine stations Helgoland and Sylt operated by the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research. J Large-Scale Res Fac 8:A184. https://doi.org/10.17815/jlsrf-8-184
Andrade SCS, Strand M, Schwartz M, Chen H, Kajihara H, von Döhren J, Sung S, Junoy J, Thiel M, Norenburg JL, Turbeville JM, Giribet G, Sundberg P (2012) Disentangling ribbon worm relationships: multi-locus analysis supports traditional classification of the phylum Nemertea. Cladistics 28:141–159. https://doi.org/10.1111/j.1096-0031.2011.00376.x
Andrade SC, Montenegro H, Strand M, Schwartz ML, Kajihara H, Norenburg JL, Turbeville JM, Giribet G (2014) A transcriptomic approach to ribbon worm systematics (Nemertea): resolving the Pilidiophora problem. Mol Biol Evol 31:3206–3215. https://doi.org/10.1093/molbev/msu253
Asa C, Phillips DM, Stover J (1986) Ultrastructure of spermatozoa of the crested tinamou. J Ultrastruct Mol Struct Res 94:170–175. https://doi.org/10.1016/0889-1605(86)90063-7
Birkhead TR, Montgomerie R (2009) Three centuries of sperm research. In: Birkhead TR, Hosken DJ, Pitnick S (eds) Sperm biology: an evolutionary perspective. Academic Press, San Diego, pp 1–42. https://doi.org/10.1016/B978-0-12-372568-4.00001-X
Buckland-Nicks J, Lundin K, Wallberg A (2019) The sperm of Xenacoelomorpha revisited: implications for the evolution of early bilaterians. Zoomorphology 138:13–27. https://doi.org/10.1007/s00435-018-0425-8
Camargo TR, Rossi N, Castilho AL, Costa RC, Mantelatto FL, Zara FJ (2016) Integrative analysis of sperm ultrastructure and molecular genetics supports the phylogenetic positioning of the sympatric rock shrimps Sicyonia dorsalis and Sicyonia typica (Decapoda, Sicyoniidae). Zoomorphology 135:67–81. https://doi.org/10.1007/s00435-015-0287-2
Campos A, Introíni GO, Tallarico LDF, Passos FD, Machado FM, Recco-Pimentel SM (2018) Ultrastructure of the spermatozoa of three species of Anomalodesmata (Mollusca, Bivalvia) and phylogenetic implications. Acta Zool. https://doi.org/10.1111/azo.12282
Chernyshev AV (2021) An updated classification of the phylum Nemertea. Invertebr Zool 18:188–196. https://doi.org/10.15298/invertzool.18.3.01
Chernyshev AV, Polyakova NE (2018) Nemerteans from deep-sea expedition SokhoBio with description of Uniporus alisae sp. nov. (Hoplonemertea: Reptantia s.l.) from the Sea of Okhotsk. Deep Sea Res Part II 154:121–139. https://doi.org/10.1016/j.dsr2.2017.09.022
Chernyshev AV, Polyakova NE (2019) Nemerteans from the deep-sea expedition KuramBio II with descriptions of three new hoplonemerteans from the Kuril-Kamchatka Trench. Prog Oceanogr 178:102148. https://doi.org/10.1016/j.pocean.2019.102148
Chernyshev AV, Polyakova NE (2022) Nemerteans collected in the Bering Sea during the research cruises aboard the R/V Akade-mik M.A. Lavrentyev in 2016, 2018, and 2021 with an analysis of deep-sea heteronemertean and hoplonemertean species. Deep-Sea Research Part II Top Stud Oceanogr 199:105081. https://doi.org/10.1016/j.dsr2.2022.105081
Chernyshev AV, Polyakova NE, Turanov SV, Kajihara H (2018) Taxonomy and phylogeny of Lineus torquatus and allies (Nemertea, Lineidae) with descriptions of a new genus and a new cryptic species. Syst Biodivers 16:55–68. https://doi.org/10.1080/14772000.2017.1317672
Chernyshev AV, Neznanova SY, Yurchenko OV (2020) Spermatozoa ultrastructure of two basal pilidiophoran nemerteans, Hubrechtella juliae and Sonnenemertes cantelli (Nemertea, Pilidiophora). Micron 133:102853. https://doi.org/10.1016/j.micron.2020.102853
Chernyshev AV, Polyakova NE, Hiebert TC, Maslakova SA (2021a) Evaluation of the taxonomic position of the genus Carinina (Nemertea: Palaeonemertea), with descriptions of two new species. Inverteb Syst 35:245–260. https://doi.org/10.1071/IS20061
Chernyshev AV, Polyakova NE, Norenburg JL, Kajihara H (2021b) A molecular phylogeny of Tetrastemma and its allies (Nemertea, Monostilifera). Zoolog Scr 50:824–836. https://doi.org/10.1111/zsc.12511
Chernyshev AV, Polyakova NE, Sun SC (2022) Phylogenetic relationships and taxonomic position of the ribbon worms of the genus Parahubrechtia (Nemertea, Palaeonemertea) with descriptions of two new species. Zool Stud 61:e38. https://doi.org/10.6620/ZS.2022.61-38
Christina, Sagorny Jörn von, Döhren Greg W., Rouse Ekin, Tilic (2022) Cutting the ribbon: bathyal Nemertea from seeps along the Costa Rica margin with descriptions of 2 new genera and 9 new species. European Journal of Taxonomy 845: 132–174. https://doi.org/10.5852/ejt.2022.845.1959
Dallai R (2014) Overview on spermatogenesis and sperm structure of Hexapoda. Arthropod Struct Dev 43:257–290. https://doi.org/10.1016/j.asd.2014.04.002
Dallai R, Gottardo M, Beutel RG (2016) Structure and evolution of insect sperm: new interpretations in the age of phylogenomics. Annu Rev Entomol 61:1–23. https://doi.org/10.1146/annurev-ento-010715-023555
Döhren J von (2009) Zur Phylogenie der Nemertea: Vergleichende Untersuchungen der Reproduktion und Entwicklung. Dissertation, Freie Universität Berlin. https://doi.org/10.17169/refubium-18092
Du Plessis L, Soley JT (2014) A re-evaluation of sperm ultrastructure in the emu, Dromaius novaehollandiae. Theriogenology 81:1073–1084. https://doi.org/10.1016/j.theriogenology.2014.01.034
Egan EA, Anderson DT (1979) The reproduction of the entozoic nemertean Gononemertes australiensis Gibson (Nemertea: Hoplonemertea: Monostylifera)–gonads, gametes, embryonic development and larval development. Mar Freshw Res 30:661–681. https://doi.org/10.1071/MF9790661
Ehlers U (1985) Das phylogenetische System der Plathelminthes. Gustav Fischer Verlag, Stuttgart
Fawcett DW (1975) The mammalian spermatozoon. Dev Biol 44:394–436. https://doi.org/10.1016/0012-1606(75)90411-X
Ferraguti M, Erséus C (1999) Sperm types and their use for a phylogenetic analysis of aquatic clitellates. In: Dorresteijn AWC, Westheide W (eds) Reproductive strategies and developmental patterns in annelids. Developments in hydrobiology, vol 142. Springer, Dordrecht, pp 225–237. https://doi.org/10.1007/978-94-017-2887-4_13
Fitzpatrick JL, Kahrl AF, Snook RR (2022) SpermTree, a species-level database of sperm morphology spanning the animal tree of life. Sci Data 9:30. https://doi.org/10.1038/s41597-022-01131-w
Franzén Å (1956) On spermiogenesis, morphology of the spermatozoon, and biology of fertilization among invertebrates. Zoologiska Bidrag Från Uppsala 31:355–482
Franzén Å (1983) Nemertina. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of Invertebrates, vol II. Spermatogenesis and sperm function. Wiley, New York, pp 159–170
Franzén Å, Sensenbaugh T (1988) The spermatozoon of Gononemertes parasita Nemertea, (Hoplonemertea) with a note on sperm evolution in the nemerteans. Int J Invert Reprod 14:25–36. https://doi.org/10.1080/01688170.1988.10510362
Giribet G, Wheeler W (2002) On bivalve phylogeny: a high-level analysis of the Bivalvia (Mollusca) based on combined morphology and DNA sequence data. Invertebr Biol 121:271–324. https://doi.org/10.1111/j.1744-7410.2002.tb00132.x
Gonzalez-Cueto J, Escarraga-Fajardo ME, Lagos AM, Quiroga S, Castro LR (2015) The complete mitochondrial genome of Micrura ignea Schwartz & Norenburg 2005 (Nemertea: Heteronemertea) and comparative analysis with other nemertean mitogenomes. Mar Genom 20:33–37. https://doi.org/10.1016/j.margen.2015.01.003
Gottardo M, Dallai R, Mercati D, Hörnschemeyer T, Beutel RG (2016) The evolution of insect sperm− an unusual character system in a megadiverse group. J Zool Syst Evol Res 54:237–256. https://doi.org/10.1111/jzs.12136
Gu NH, Zhao WL, Wang GS, Sun F (2019) Comparative analysis of mammalian sperm ultrastructure reveals relationships between sperm morphology, mitochondrial functions and motility. Reprod Biol Endocrinol 17:66. https://doi.org/10.1186/s12958-019-0510-y
Jaana H, Yamamoto TS (1981) The ultrastructure of spermatozoa with a note on the formation of the acrosomal filament in the lamprey, Lampetra japonica. Jpn J Ichthyol 28:135–147. https://doi.org/10.11369/jji1950.28.135
Jamieson BGM (1984) Spermatozoal ultrastructure in Branchiostoma moretonensis Kelly, a comparison with B. lanceolatum (Cephalochordata) and with other deuterostomes. Zool Scr 13:223–229. https://doi.org/10.1111/j.1463-6409.1984.tb00039.x
Jamieson BGM, Rouse GW (1989) The spermatozoa of the Polychaeta (Annelida): an ultrastructural review. Biol Rev 64:93–157. https://doi.org/10.1111/j.1469-185X.1989.tb00673.x
Jamieson BGM, Dallai R, Afzelius BA (1999) Insects: their spermatozoa and phylogeny. Science Publishers Inc, Enfield
Jamieson BMG 2007 Avian spermatozoa: structure and phylogeny. In: Jamieson BGM (ed) Reproductive biology and phylogeny of birds, Vol 6A: Phylogeny, morphology, hormones and fertilization. Science Publishers, Enfield, New Hampshire (USA), pp 349-511.
Jespersen A (1994) Spermiogenesis, sperm structure and fertilization in the palaeonemertean Cephalothrix rufifrons (Nemertini, Anopla). Zoomorphology 114:119–124. https://doi.org/10.1007/BF00396644
Justine J-L (1998) Spermatozoa as phylogenetic characters for the Eucestoda. J Parasitol 84:385–408. https://doi.org/10.2307/3284502
Justine J-L (2014) Spermatozoa as phylogenetic characters for the Plathelminthes. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Plathelminthes. CRC Press, London, pp 231–238
Kahrl AF, Snook RR, Fitzpatrick JL (2021) Fertilization mode drives sperm length evolution across the animal tree of life. Nat Ecol Evol 5:1153–1164
Kajihara H (2021) Higher classification of the Monostilifera (Nemertea: Hoplonemertea). Zootaxa 4920:151–199. https://doi.org/10.11646/ZOOTAXA.4920.2.1
Kajihara H, Abukawa S, Chernyshev AV (2022a) Exploring the basal topology of the heteronemertean tree of life: establishment of a new family, along with turbotaxonomy of Valenciniidae (Nemertea: Pilidiophora: Heteronemertea). Zool J Linn Soc 196:503–548. https://doi.org/10.1093/zoolinnean/zlac015
Kajihara H, Ganaha I, Kohtsuka H (2022b) Lineid heteronemerteans (Nemertea: Pilidiophora) from Sagami Bay, Japan, with some proposals for the family-level classification system. Zoolog Sci 39:62–80. https://doi.org/10.2108/zs210059
Krämer D (2017) Species identification and delimitation in Nemerteans. Dissertation, Universität Bonn. https://nbn-resolving.org/urn:nbn:de:hbz:5n-46079
Kvist S, Laumer CE, Junoy J, Giribet G (2014) New insights into the phylogeny, systematics and DNA barcoding of Nemertea. Invertebr Syst 28:287–308. https://doi.org/10.1071/IS13061
Kvist S, Chernyshev A, Giribet G (2015) Phylogeny of Nemertea with special interest in the placement of diversity from Far East Russia and northeast Asia. Hydrobiologia 760:105–119. https://doi.org/10.1007/s10750-015-2310-5
Lindemann CB, Lesich KA (2016) Functional anatomy of the mammalian sperm flagellum. Cytoskeleton 73:652–669. https://doi.org/10.1002/cm.21338
Lüpold S, Pitnick S (2018) Sperm form and function: what do we know about the role of sexual selection? Reproduction 155:R229–R243. https://doi.org/10.1530/REP-17-0536
Mattei X (1988) The flagellar apparatus of spermatozoa in fish. Ultrastructure and evolution. Biol Cell 63:151–158. https://doi.org/10.1016/0248-4900(88)90054-8
Mattei X (1991) Spermatozoon ultrastructure and its systematic implications in fishes. Can J Zool 69:3038–3055. https://doi.org/10.1139/z91-428
Morisawa S (1995) Fine structure of spermatozoa of the hagfish Eptatretus burgeri (Agnatha). Biol Bull 189:6–12. https://doi.org/10.2307/1542195
Quagio-Grassiotto I, Baicere-Silva CM, de Oliveira-Santana JC, Mirande JM (2020) Spermiogeneis and sperm ultrastructure as sources of phylogenetic characters. The example of characid fishes (Teleostei: Characiformes). Zool Anz 289:77–86. https://doi.org/10.1016/j.jcz.2020.09.006
Rouse GW (2005) Annelid sperm and fertilization biology. Hydrobiologia 535:167–178. https://doi.org/10.1007/s10750-004-4390-5
Schwartz ML (2009) Untying a Gordian knot of worms: systematics and taxonomy of the Pilidiophora (phylum Nemertea) from multiple data sets. The George Washington University ProQuest Dissertations Publishing, 3339279
Schwartz ML, Norenburg JL (2001) Can we infer heteronemertean phylogeny from available morphological data? Hydrobiologia 456:165–174. https://doi.org/10.1023/A:1013093629108
Soley JT, Du Plessis L (2020) Ultra-imaging in applied animal andrology: the power and the beauty. Anim Reprod Sci 220:106306. https://doi.org/10.1016/j.anireprosci.2020.106306
Støstad HN, Johnsen A, Lifjeld JT, Rowe M (2018) Sperm head morphology is associated with sperm swimming speed: a comparative study of songbirds using electron microscopy. Evolution 72:1918–1932
Stricker SA, Cavey MJ (1986) An ultrastructural study of spermatogenesis and the morphology of the testis in the nemertean worm Tetrastemma phyllospadicola (Nemertea, Hoplonemertea). Can J Zool 64:2187–2202. https://doi.org/10.1139/z86-333
Stricker SA, Folsom MW (1998) A comparative ultrastructural analysis of spermatogenesis in nemertean worms. Hydrobiologia 365:55–72. https://doi.org/10.1023/A:1003186611254
Sundberg P, Saur M (1998) Molecular Phylogeny of Some European Heteronemertean (Nemertea) Species and the Monophyletic Status of Riseriellus, Lineus, and Micrura. Mol Phylogenet Evol 10:271–280. https://doi.org/10.1006/mpev.1998.0543
Thollesson M, Norenburg JL (2003) Ribbon worm relationships: A phylogeny of the phylum Nemertea. Proc R Soc Lond B Biol Sci 270:407–415. https://doi.org/10.1098/rspb.2002.2254
Tudge C (2009) Spermatozoal morphology and its bearing on decapod phylogenetics. In: Martin JW, Crandal KA, Felder DL (eds) Decapod crustacean phylogeny. CRC Press Taylor & Francis group, Boca Raton, pp 113–132
Turbeville JM, Ruppert EE (1985) Comparative ultrastructure and the evolution of nemertines. Am Zool 25:53–71. https://doi.org/10.1093/icb/25.1.53
Valchi P, Ponssa ML, Farías A, Volonteri MC, Hermida GN (2023) Comparative spermatozoa ultrastructure of neotropical grass frogs (genus Leptodactylus) with comments on anuran reproductive modes and phylogeny. Zool Anz 302:188–185
Van Deurs B, Lastein U (1973) Ultrastructure of the spermatozoa of the teleost Pantodon buchholzi Peters, with particular reference to the midpiece. J Ultrastruct Res 42:517–533. https://doi.org/10.1016/S0022-5320(73)80024-3
von Döhren J (2016) First record on the development of the larva of the basally branching nemertean species Carinina ochracea (Palaeonemertea). Helgol Mar Res 70:13. https://doi.org/10.1186/s10152-016-0466-7
von Döhren J, Bartolomaeus T (2006) Ultrastructure of sperm and male reproductive system in Lineus viridis (Heteronemertea, Nemertea). Zoomorphology 125:175–185. https://doi.org/10.1007/s00435-006-0024-y
von Döhren J, Beckers P, Vogeler R, Bartolomaeus T (2010) Comparative sperm ultrastructure in Nemertea. J Morphol 271:793–813. https://doi.org/10.1002/jmor.10834
Whitfield PJ (1972) The ultrastructure of the spermatozoon of the hoplonemertine, Emplectonema neesii. Z Zellforsch 128:303–316. https://doi.org/10.1007/BF00306972
Yurchenko OV, Chernyshev AV (2022) Sperm morphology and some aspects of acrosomal complex development in four species of Heteronemertea (Pilidiophora, Nemertea). Zool Anz 299:38–48. https://doi.org/10.1016/j.jcz.2022.05.005
Yurchenko OV, Neznanova SY, Chernyshev AV (2021) A comparative morphological study of the testes, spermatogenesis, and spermatozoa in two nemertean species, Callinera sp. and Parahubrechtia sp. (Palaeonemertea, Tubulanidae). Zool Anz 294:114–127. https://doi.org/10.1016/j.jcz.2021.08.005
Acknowledgements
First of all, we want to thank the staff of the Station de Biologie Marine (Concarneau, France) for providing lab space and continuous support. Our thanks are also due to staffs of the AWI Wadden Sea Station Sylt (Germany), the Station Biologique de Roscoff (France) and the Laboratoire Arago (Banyuls-sur-Mer, France). The research leading to these results received fundings from the European Union’s 7th Framework Program “Capacities” Research Infrastructures under grant agreement 227799 for the Assemble Marine project “Comparative Development in Nemerteans (NeCD)” and “EvoDevo of Polystilifera (EvoDevoPol)”, granted to Jörn von Döhren. It also received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 730984 for the ASSEMBLE Plus projects "Evolutionary Developmental Biology of Palaeonemertea (EvoDevoPal)”, also granted to Jörn von Döhren. The study also received funding from the Alfred-Wegener-Institut Helmholtz-Zentrum (AWI_BAH_03). Technical infrastructure to perform the study was provided by the DFG (INST 217/1072-1 FUGG). Last, but not least, we would like to thank Tatjana Bartz for technical assistance during the study as well as two anonymous reviewers for improving the quality of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
T.B. and J.vD wrote the main manuscript, V.B., T.B., JvD and L.A. did the electron microscopical analyses, T.B. did the reconstructions and prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bartolomaeus, T., Bronkars, V., Adam, L. et al. Ultrastructure and phylogenetic significance of spermatozoa in Nemertea. Zoomorphology (2024). https://doi.org/10.1007/s00435-024-00659-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00435-024-00659-2