Abstract
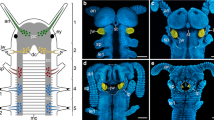
This study describes the innervation patterns for the cerebral nerves which project to the cephalic sensory organs (CSOs) in the opisthobranch Haminoea hydatis (Linnaeus 1758) and uses axonal tracing techniques (backfilling) to reveal the central cellular origins for these cerebral nerves. Cell clusters projecting into the cerebral nerves can be defined by their positions in the ganglion relative to other clusters, nerve roots and lobes. The number of cell clusters and the relative sizes of somata are constant in a given cluster, whereas the absolute number of somata and absolute sizes of single somata in a given cluster increase with the size of the animal. Additionally, the invariable morphological characteristics of the cell clusters are used to define criteria for the assessment of possible homology for the clusters innervating the CSOs in Opisthobranchia. The data suggest two different strategies to accommodate the increasing body size; first, the additions of nerve cells and second, the growth of nerve cells.







Similar content being viewed by others
References
Altman JS, Tyrer NM (1980) Filling selected neurons with cobalt through cut nerves. In: Strausfeld NJ, Miller TA (eds) Neuroanatomical techniques: insect nervous system. Springer, New York, pp 357–372
Arbas EA (1991) Evolution in nervous systems. Annu Rev Neurosci 14:9–38. doi:10.1146/annurev.ne.14.030191.000301
Audesirk TE (1979) Oral mechanoreceptors in Tritonia diomedea. J Comp Physiol 130:71–78. doi:10.1007/BF02582975
Bicker G, Davis WJ, Matera EM (1982) Chemoreception and mechanoreception in the gastropod mollusc Pleurobranchea californica. J Comp Physiol 149:235–250. doi:10.1007/BF00619217
Boudko DY, Switzer-Dunlap M, Hadfield MG (1999) Cellular and subcellular structure of anterior sensory pathways in Phestilla sibogae (Gastropda, Nudibranchia). J Comp Neurol 403:39–52. doi:10.1002/(SICI)1096-9861(19990105)403:1<39::AID-CNE4>3.0.CO;2-B
Cash D, Carew TJ (1989) A quantitative-analyses of the development of the central nervous system in juvenile Aplysia californica. J Neurobiol 20(1):25–47. doi:10.1002/neu.480200104
Chase R (2002) In: Behavior and its neural control in gastropod molluscs. Oxford University Press, Oxford
Chester CM (1993) Comparative feeding biology of Acteocina canaliculita (SAY, 1826) and Haminoea solitaria (SAY, 1822) (Opisthobranchia, Cephalaspidea). Am Mal Bull 10(1):93–101
Chiasson BJ, Baker MW, Croll RP (1994) Morphological changes and functional recovery following axotomy of a serotonergic cerebrobuccal neurone in the land snail Achatina fulica. J Exp Biol 192:147–167
Coleman CO (2003) “Digital inking”. How to make perfect line drawings on computers. Organisms, Diversity and Evolution, Electronic Supplement. 14, 1–14, http://senckenberg.de/odes/03-14.htm
Croll RP (1983) Gastropod chemoreception. Biol Rev Camb Philos Soc 58(Suppl 3):293–319. doi:10.1111/j.1469-185X.1983.tb00391.x
Croll RP (1987) Identified neurons and cellular homologies. In: Ali MA (ed) Nervous systems in invertebrates. Springer, New York, pp 41–59
Croll RP, Chiasson BJ (1989) Postembryonic development of serotoninlike immunoreactivity in the central nervous system of the snail, Lymnaea stagnalis. J Comp Neurol 280:122–142. doi:10.1002/cne.902800109
Croll RP, Baker M (1990) Axonal regeneration and sprouting following injury to the cerebral-buccal connective in the snail Achatina fulica. J Comp Neurol 300:273–286. doi:10.1002/cne.903000210
Croll RP, Boudko DY, Pires A, Hadfield MG (2003) Transmitter content of cells and fibers in the cephalic sensory organs of the gastropod mollusc Phestilla sibogae. Cell Tissue Res 314:437–448. doi:10.1007/s00441-003-0778-1
Davis WJ, Matera EM (1982) Chemoreception in gastropod molluscs: electron microscopy of putative receptor cells. J Neurobiol 13(1):79–84. doi:10.1002/neu.480130109
Dayrat B, Tillier S (2002) Evolutionary relationships of euthyneuran gastropods (Mollusca): a cladistic re-evaluationof morphological characters. Zool J Linn Soc 135:403–470. doi:10.1046/j.1096-3642.2002.00018.x
Edlinger K (1980) Zur Phylogenie der chemischen Sinnesorgane einiger Cephalaspidea (Mollusca, Opisthobranchia). Zeitschrift für Zoologie. Systematik Evolutionsforschung 18:241–256
Elliott CJH, Susswein AJ (2002) Comparative neuroethology of feeding control in molluscs. J Exp Biol 205(7):877–896
Emery DG (1992) Fine structure of olfactory epithelia of gastropod molluscs. Microsc Res Tech 22:307–324. doi:10.1002/jemt.1070220402
Fredman SM (1987) Intracellular staining of neurons with nickel-lysine. J Neurosci Methods 20(3):181–194. doi:10.1016/0165-0270(87)90050-1
Goodman CS, Pearson KG, Heitler WJ (1979) Variability of identified neurons in grasshoppers. Comp Biochem Physiol 64A:455–462. doi:10.1016/0300-9629(79)90571-1
Gosliner TM (1994) Gastropoda: Opisthobranchia. In: Harrisson FE, Kohn AJ (eds) Microscopic anatomy of invertebrates, 5: Mollusca. Wiley, New York, pp 253–355
Hauser M, Koopowitz H (1987) Age-dependent changes in fluorescent neurons in the brain of Notoplana acticola, a polyclad flatworm. J Exp Zool 241:217–225. doi:10.1002/jez.1402410208
Hayman-Paul D (1991) Pedigrees of neurobehavioral circuits: tracing the evolution of novel behaviors by comparing motor patterns, muscles, and neurons in members of related taxa. Brain Behav Evol 38:226–239. doi:10.1159/000114390
Hoffmann H (1939) Mollusca. I Opisthobranchia. In: Bronns HG (ed) Klassen und Ordnungen des Tierreichs III (1). Akademische-Verlagsgesellschaft, Leipzig, pp 1–1248
Hubendick B (1951) Recent Lymneidae: their variation, morphology, taxonomy, nomenclature and distribution. Almquist Wiksells Boktryckeriab, Stockholm
Huber G (1993) On the cerebal nervous system of marine heterobranchia (Gastropoda). J Molluscan Stud 59:381–420. doi:10.1093/mollus/59.4.381
Kerkhoven RM, Croll RP, Van Minnen J, Bogerd J, Ramkema MD, Lodder H et al (1991) Axonal mapping of the giant peptidergic neurons VD1 and RPD2 located in the CNS of the pond snail Lymnaea stagnalis, with particular reference to the innervation of the auricle of the heart. Brain Res 565:8–16. doi:10.1016/0006-8993(91)91730-O
Kutsch W, Breidbach O (1994) Homologous structures in the nervous systems of Arthropoda. Adv Insect Physiol 24:2–113
Newcomb JM, Fickbohm DJ, Katz PS (2006) Comparative mapping of serotonin-immunoreactive neurons in the central nervous system of nudibranch molluscs. J Comp Neurol 499:485–505. doi:10.1002/cne.21111
Ogawa F (1939) The nervous system of earthworm (Pheretima communissima) in different ages. Science reports of the Tohoku Imperial University (Series 4) 8:395–488
Salvini-Plawen L, Steiner G (1996) Synapomorphies and plesiomorphies in higher classification of mollusca. In: Taylor J (ed) Origin and evolutionary radiation of the Mollusca. Oxford University Press, Oxford, pp 29–51
Stewart RR, Spergel D, Macagno ER (1986) Segmental differentiation in the leech nervous system: the genesis of cell number in the segmental ganglia of Haemopsis marmorata. J Comp Neurol 253:253–259. doi:10.1002/cne.902530211
Acknowledgments
We wish to thank Claudia Nesselhauf for her help to build up the laboratory population of H. hydatis. The Aquazoo Düsseldorf and Dr. Ulrike Schulte-Oehlmann kindly provided the first animals. This study was supported by the German Science Foundation, KL 1303/3-1 and by the Verein der Freunde und Förderer der Johann-Wolfgang-Goethe Universität.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below are the links to the electronic supplementary material.
435_2008_64_MOESM1_ESM.xls
Table of the number of specimen, shell size calculated by the product of length and breadth in mm2 and maximum diameter of somata (in μm) within the cerebral clusters projecting into the N2 (27.5 kb)
Rights and permissions
About this article
Cite this article
Staubach, S., Schützner, P., Croll, R.P. et al. Innervation patterns of the cerebral nerves in Haminoea hydatis (Gastropoda: Opisthobranchia): a test for intraspecific variability. Zoomorphology 127, 203–212 (2008). https://doi.org/10.1007/s00435-008-0064-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-008-0064-6