Abstract
Purpose
Treatment recommendations for patients with polycythemia vera (PV) are well established. Most multicenter trials investigating novel therapeutic strategies for PV are developed and conducted at university hospitals and specialized academic centers. The majority of patients in Germany, however, are treated in an outpatient (ambulatory) setting. The aim of this study was to evaluate the ‘real-life’ situation in a cohort of 1467 patients by analyzing data from a survey conducted at private practices and primary care centers.
Methods
Eligible private practices and primary care centers treating patients with MPN were recruited to participate in a paper–pencil-based survey conducted from March 2015 until March 2016 in Germany. Hematologists were asked to report from patient charts. Descriptive analyses were conducted to assess for outcomes examined by reported prognostic risk scores, symptom scores and clinical response criteria.
Results
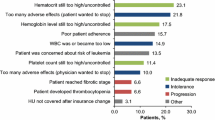
In total, 34 centers participated in our retrospective survey and provided data on 1476 patients. Most patients were of older age (66.7 % older than 66 years of age), which was the main risk factor according to the criteria published by Tefferi and colleagues. Molecular status at diagnosis was not evaluated in 23 % of patients. Low rates of constitutional symptoms were reported in this physician-based survey with concentration problems, fatigue and itching being the main PV-related symptoms. Phlebotomy and hydroxyurea were the main cytoreductive measures for hematocrit control. The majority of patients were responsive (67.8 %) and tolerant (77.3 %) to hydroxyurea therapy. Interferon and JAK inhibitor therapy were used in <10 % of patients, respectively. Overall, leukocytosis, thrombocytosis and hematocrit could be effectively controlled by any therapy applied. Lack of efficacy was reported on reduction of constitutional symptoms and splenomegaly.
Conclusions
The patient population investigated was older than participants in published large multicenter trials. The majority of patients were categorized as ‘high risk.’ Age was the main risk factor. Molecular status was unavailable in the majority of patients diagnosed prior to 2008. The physician-based survey reported on significantly lower rates of constitutional symptoms than patient-based surveys in the literature. Consistent with previously published reports, hydroxyurea is the main agent used for PV therapy in an outpatient setting resulting in efficient control of hematopoietic parameters. Constitutional symptoms and splenomegaly, however, may not be reduced efficiently, which could be improved by the use of JAK inhibitor treatment for high-risk patients in the future.






Similar content being viewed by others
References
Antonioli E, Guglielmelli P, Pieri L, Finazzi M, Rumi E, Martinelli V et al (2012) Hydroxyurea-related toxicity in 3,411 patients with Ph’-negative MPN. Am J Hematol 87:552–554
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al (2016) The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391–2405
Barbui T, Thiele J, Vannucchi AM, Tefferi A (2015) Rationale for revision and proposed changes of the WHO diagnostic criteria for polycythemia vera, essential thrombocythemia and primary myelofibrosis. Blood Cancer J 5:e337
Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC et al (2009) Response criteria for essential thrombocythemia and polycythemia vera: result of a European leukemianet consensus conference. Blood 113:4829–4833
Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch H et al (2010) A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European leukemianet (ELN) consensus process. Br J Haematol 148:961–963
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S et al (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054–1061
Chievitz E, Thiede T (1962) Complications and causes of death in polycythaemia vera. Acta Med Scand 172:513–523
Diehn F, Tefferi A (2001) Pruritus in polycythaemia vera: prevalence, laboratory correlates and management. Br J Haematol 115:619–621
Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF et al (2014) Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 371:2477–2487
Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA et al (2015) Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood 126:1762–1769
Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D et al (2005) Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 353:33–45
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG et al (2014) Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371:2488–2498
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C et al (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434:1144–1148
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H et al (2016) Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest 149:315–352
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352:1779–1790
Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C et al (2004) Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 350:114–124
Lengfelder E, Baerlocher GM, Gisslinger HMG and Petrides PE (2014) Polycythaemia vera (PV). Onkopedia-Leitlinie der Deutschen Gesellschaft für Hämatologie und Onkologie (DGHO)
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ et al (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7:387–397
Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D et al (2013) Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 368:22–33
Mesa R, Miller CB, Thyne M, Mangan J, Goldberger S, Fazal S et al (2014) Impact of myeloproliferative neoplasms (mpns) on patients’ overall health and productivity: results from the MPN landmark survey in the United States. Blood—ASH annual meeting abstracts; Abstract 3183
Mesa R, Miller CB, Thyne M, Mangan J, Goldberger S, Fazal S et al (2016) Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN landmark survey. BMC Cancer 16:167
Mossuz P, Girodon F, Donnard M, Latger-Cannard V, Dobo I, Boiret N et al (2004) Diagnostic value of serum erythropoietin level in patients with absolute erythrocytosis. Haematologica 89:1194–1198
Parasuraman S, DiBonaventura M, Reith K, Naim A, Concialdi K, Sarlis NJ (2015) Patterns of hydroxyurea use and clinical outcomes among patients with polycythemia vera in real-world clinical practice: a chart review. Exp Hematol Oncol 5:3
Passamonti F, Griesshammer M, Palandri F, Egyed M, Benevolo G, Devos T et al (2016) Ruxolitinib proves superior to best available therapy in patients with polycythemia vera resistant to or intolerant of hydroxyurea without splenomegaly: results from response-2. Haematologica (EHA annual meeting abstracts)
Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM et al (2011) The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 118:401–408
Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR et al (2007) JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 356:459–468
Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F et al (2013) Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 27:1874–1881
Vannucchi AM (2014) How I treat polycythemia vera. Blood 124:3212–3220
Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F et al (2015) Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 372:426–435
Acknowledgments
We thank all the participating centers for their contributions and Dres. Kiewe (Berlin), Mohr (Hamburg), Sauer (Potsdam) and Prof. Josting (Berlin) for discussion of the scientific data.
Funding
Kathleen Jentsch-Ullrich received research funding for this project from Novartis Inc. (Germany).
Author contributions
Judith Eberhardt, Vanja Zeremski, Michael Köhler and Denise Wolleschak analyzed data. Kathleen Jentsch-Ullrich and Florian H. Heidel analyzed data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Florian H. Heidel is advisory board member for Novartis Inc. (Germany).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. In this survey, no identifiable personal information or patient data were reported by the participating physicians. Questionnaires and study materials were reviewed by the institutional review board.
Rights and permissions
About this article
Cite this article
Jentsch-Ullrich, K., Eberhardt, J., Zeremski, V. et al. Characteristics and treatment of polycythemia vera patients in clinical practice: a multicenter chart review on 1476 individuals in Germany. J Cancer Res Clin Oncol 142, 2041–2049 (2016). https://doi.org/10.1007/s00432-016-2209-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2209-1