Abstract
Background
The Philadelphia chromosome−negative myeloproliferative neoplasms (MPN) myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET) negatively affect patient quality of life (QoL) and are associated with increased risk of mortality.
Methods
The MPN Landmark survey was conducted from May to July 2014 in patients with MF, PV, or ET under active management in the United States. The survey assessed respondent perceptions of disease burden and treatment management and included questions on overall disease burden, QoL, activities of daily living, and work productivity. Outcomes were further analyzed by calculated (ie, not respondent-reported) prognostic risk score and symptom severity quartile.
Results
The survey was completed by 813 respondents (MF, n = 207; PV, n = 380; ET, n = 226). The median respondent age in each of the 3 MPN subtypes ranged from 62 to 66 years; median disease duration was 4 to 7 years. Many respondents reported that they had experienced MPN-related symptoms ≥1 year before diagnosis (MF, 49 %; PV, 61 %; ET, 58 %). Respondents also reported that MPN-related symptoms reduced their QoL, including respondents with low prognostic risk scores (MF, 67 %; PV, 62 %; ET, 57 %) and low symptom severity (MF, 51 %; PV, 33 %; ET, 15 %). Many respondents, including those with a low prognostic risk score, reported that their MPN had caused them to cancel planned activities or call in sick to work at least once in the preceding 30 days (cancel planned activities: MF, 56 %; PV, 35 %; ET, 35 %; call in sick: MF, 40 %; PV, 21 %; ET, 23 %).
Conclusions
These findings of the MPN Landmark survey support previous research about the symptom burden experienced by patients with MPNs and are the first to detail the challenges that patients with MPNs experience related to reductions in activities of daily living and work productivity.
Similar content being viewed by others
Background
Myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET) are Philadelphia chromosome−negative myeloproliferative neoplasms (MPNs) that are frequently associated with the JAK2 V617F mutation [1]. Presentations and symptom profiles of these MPNs vary with subtype but often include erythrocytosis, thrombocytosis, leukocytosis, and/or splenomegaly [1, 2]. Prevalence of PV and ET is approximately 10 times higher than MF; prevalence per 100,000 residents in the United States (2008–2010) was 4 to 6 people for MF, 45 to 57 for PV, and 39 to 57 for ET [3].
Patients with MPNs experience a broad array of symptoms that negatively affect their quality of life (QoL) [2]. Symptoms often include fatigue, concentration problems, night sweats, pruritus, and splenomegaly-related symptoms (eg, early satiety and abdominal discomfort and/or pain) [4, 5]. In addition, patients with MF, PV, or ET have increased risk of mortality compared with the general population [6]. Cardiovascular events and fibrotic and/or leukemic transformation are important causes of morbidity and mortality in patients with MPNs [7–9]. One study reported that median survival is approximately 6 years for patients with MF (median age at diagnosis, 63 years; median follow-up, 8 years); 14 years for patients with PV (median age at diagnosis, 64 years; median follow-up, 12 years); and 20 years for patients with ET (median age at diagnosis, 55 years; median follow-up, 17 years) [10].
Numerous questions remain regarding myeloproliferative disease burden and management. A limited amount of data about the extent to which MPNs affect activities of daily living are available in the published literature [11], and we are unaware of any published reports about the productivity of patients with MPNs who are employed. Methods for identifying high-risk patients have been developed based on known risk factors [12–14] and symptom severity [15]. Prognostic risk score models have been proposed for MF [12], PV [13], and ET [14]; however, the predictive value of these systems for identifying comprehensive patient burden that includes QoL and productivity impairments has not been evaluated.
The MPN Landmark survey evaluated the patient disease burden in the Philadelphia chromosome–negative MPN disease setting. This first analysis of the MPN Landmark survey includes data concerning the impact of MPNs on health and productivity as reported by a contemporary population of respondents with MPNs in the United States.
Methods
Respondents
Patients in the United States with a previous diagnosis of MF, PV, or ET were eligible to take the survey. Respondents were recruited through physician offices, advocacy groups, and the media. Invitations to complete a web-based survey were delivered by direct mail to patients nationwide. Digital recruiting was conducted online at 50 websites and a print ad campaign was conducted across 13 newspapers in 5 major metropolitan regions (Chicago, IL; Dallas, TX; Houston, TX; Philadelphia, PA; and New York, NY). To supplement the multichannel recruitment effort, 1500 additional invitations were distributed through specialists who were treating patients with MPNs. Surveys were administered online and completed between May and July 2014; respondents did not receive remuneration for participating. Investigators were blinded to the method by which individual respondents were recruited, and the survey did not ask respondents to report their recruitment method.
Survey instrument
A web-based survey that included 65 multiple-choice questions with an estimated completion time of 20 to 25 minutes was presented to each respondent. A summary of the patient respondent portion of the MPN Landmark survey is included in the Additional file 1: Respondents answered questions tailored to their diagnosis—MF, PV, or ET. The current report includes observational findings from respondents based on questions related to (1) respondent demographics; (2) disease features; (3) symptom burden; (4) disease burdens related to QoL, activities of daily living, and work productivity; and (5) treatment management and therapies. Individual MPN-related symptoms were evaluated using an adapted version of the MPN Symptom Assessment Form (MPN-SAF) [5] and were rated on a scale that ranged from 0 (absent) to 10 (worst imaginable); based on the structure of the scale, individual symptoms with severity scores ≥7 were considered very severe. Questions evaluating emotional impact and burden of disease were evaluated on a scale that ranged from 1 (not at all) to 5 (a great deal). Comorbidities were based on respondent answers to questions about “current medical conditions” and were not confirmed with medical records (eg, “leukemia” could represent any leukemia subtype or other blood disorders that respondents correctly or incorrectly considered to be leukemia).
Statistical methods
Study findings were analyzed using descriptive statistics. The survey included a core set of mandatory questions that required answers before completion. Analyses based on optional questions excluded all respondents who did not provide an answer. Respondent outcomes were examined by respondent-reported symptom severity quartile and calculated prognostic risk scores. To identify trends related to the effect of MPN symptom severity on activities of daily living and work productivity, respondents were stratified by disease-related symptom severity quartile using abbreviated (10-item) MPN-SAF total symptom scores (MPN-SAF TSS) [4]. Previous studies of MPN-SAF TSS quartiles in patients with MPNs found that higher quartiles were associated with increased measures of disease severity (eg, presence of cytopenias, prior thrombosis, individual symptom scores) [15, 16]. On a scale of 0 (absent) to 100 (worst imaginable), symptom severity quartiles were determined after survey data collection in an effort to include similar numbers of respondents in each quartile and were defined as follows: quartile 1, 0–5; quartile 2, 6–13; quartile 3, 14–26; quartile 4, 27–78. Respondents were also stratified by prognostic risk scores to identify trends related to the effects of risk scores on activities of daily living and work productivity. Prognostic risk scores were calculated based on information provided by respondents regarding medical history events and laboratory values at any point between diagnosis and the time of the survey and were generated using published scoring systems for MF (Dynamic International Prognostic Scoring System) [12], PV (modified from Tefferi et al.) [13], and ET (International Prognostic Score for Essential Thrombocythemia) [14] (Additional file 1: Table S1). Respondents who could not recall required information for prognostic risk score calculations were excluded from the related subgroup analyses. This report analyzed quartile 1 and quartile 4 symptom severity subgroups and low- and high-risk prognostic risk score subgroups in an effort to focus on (1) respondents with potentially underappreciated MPN disease severity (ie, those with the lowest symptom severity or low prognostic risk) and (2) respondents with the potentially greatest disease severity (ie, those with the highest symptom severity and/or high prognostic risk).
Ethics, consent and permissions
The study received approval from the ICF International internal ethics review board, which is registered with the US Department of Health and Human Services Office of Human Research Protections and has Federalwide Assurance (FWA #00000845; ethics review board chair, Janet Griffith, PhD). ICF International assisted in conducting the MPN Landmark survey. All respondents provided informed consent.
Results
Respondent demographics
The survey was completed by 813 respondents (MF, n = 207; PV, n = 380; ET, n = 226), representing 47 states and the District of Columbia. Most respondents were women; 98 % were white (Table 1). The median age was similar between groups (MF, 66 years; PV, 64 years; ET, 62 years), and a subgroup of respondents were <50 years of age at diagnosis (MF, 19 %; PV, 27 %; ET, 43 %). Nearly all respondents reported being covered by some form of health insurance, predominantly group commercial insurance or Medicare. A subset of respondents reported that they relied on a caregiver (either “rarely,” “sometimes,” or “often”) because of their MPN (MF, 41 %; PV, 22 %; ET, 15 %).
Disease features
Median disease durations for respondents with MF, PV, and ET were 4 years, 7 years, and 7 years, respectively (Table 1). Most respondents had an intermediate or high prognostic risk score calculated using information collected during the survey and previously published scoring systems described in Additional file 1: Table S1.
A subgroup of respondents had comorbidities at the time of the survey (MF, 44 %; PV, 37 %; ET, 37 %). The most frequently reported comorbidities for respondents with MF were diabetes (6 %), moderate to severe kidney disease (6 %), emphysema/chronic obstructive pulmonary disease (COPD)/chronic bronchitis (5 %), and leukemia (5 %); for respondents with PV, diabetes (7 %), connective tissue disorders (6 %), moderate to severe kidney disease (5 %), and emphysema/COPD/chronic bronchitis (5 %); and for respondents with ET, moderate to severe kidney disease (5 %), myocardial infarction (4 %), diabetes (4 %), solid tumor (4 %), and narrowing and hardening of the arteries to the limbs (4 %).
Symptom burden
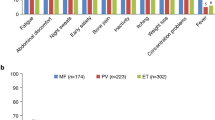
In agreement with other reports in patients with MPNs [4, 5, 18], the MPN Landmark survey respondent population reported a broad symptom burden; Table 2 presents the mean scores and incidences of symptoms included in the MPN-SAF TSS for respondents who experienced symptoms within the last 12 months. Among respondents with MF, the mean MPN-SAF TSS was more severe in those with higher versus lower calculated prognostic risk scores (MPN-SAF TSS in highest vs lowest prognostic risk category, 30.8 vs 8.1). However, this trend was not observed among respondents with PV (MPN-SAF TSS in highest vs lowest prognostic risk category, 16.2 vs 16.8) or ET (13.1 vs 18.1, respectively). A subset of respondents reported that their symptoms were very severe (ie, severity score of ≥7 out of 10; Additional file 1: Fig. S1). Most respondents had symptoms at diagnosis (MF, 78 %; PV, 88 %; ET, 81 %), with fatigue being the most frequently reported.
Many respondents reported that ≥1 MPN-related symptom manifested ≥1 year before diagnosis (MF, 49 %; PV, 61 %; ET, 58 %). Notable proportions of respondents with MF reported that fatigue (29 %) and difficulty sleeping (15 %) manifested ≥1 year before diagnosis. Respondents with PV and ET reported that the most common symptoms to manifest ≥1 year before diagnosis were fatigue (26 % and 23 %, respectively) and headaches (16 % and 16 %).
Quality of life, activities of daily living, and work productivity
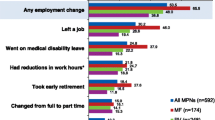
Many respondents reported that their MPN-related symptoms reduced their QoL (MF, 81 %; PV, 66 %; ET, 57 %). Reduced QoL due to MPN-related symptoms was self-reported even by respondents with low calculated prognostic risk scores (MF, 67 %; PV, 62 %; ET, 57 %) and those in the lowest symptom severity quartile (MF, 51 %; PV, 33 %; ET, 15 %; Fig. 1).
Impact of MPNs on QoL, work, and activities of daily living. MPN impact was stratified by calculated prognostic risk score and symptom severity quartile in respondents with (a) MF, (b) PV, and (c) ET. ET = essential thrombocythemia; MF = myelofibrosis; MPN = myeloproliferative neoplasm; PV = polycythemia vera; Q1 = quartile 1; Q4 = quartile 4; QoL = quality of life. * ≥ 1 day in the preceding 30 days
A notable proportion of respondents reported that their MPN interfered with activities of daily living (Table 3). Many respondents (≥45 %) in each group reported that their activities were limited by pain/discomfort; some respondents (MF, 12 %; PV, 10 %; ET, 7 %) reported that this occurred “a great deal” (Table 3). More than 10 % of respondents in each group reported that their MPN caused the cancelation of planned activities in ≥4 of the preceding 30 days (Table 3). Among respondents with low prognostic risk scores, ≥35 % reported canceling ≥1 day of planned activities in the preceding 30 days because of their condition (Fig. 1). Most respondents reported feeling anxious or worried about their MPN (MF, 91 %; PV, 78 %; ET, 74 %) and 63 % of respondents with PV reported stress/anxiety related to managing their hematocrit at <45 %. Many respondents reported feeling depressed (MF, 75 %; PV, 60 %; ET, 59 %) and/or angry (MF, 43 %; PV, 38 %; ET, 38 %). Some respondents had difficulty coping with stress (MF, 50 %; PV, 46 %; ET, 43 %). Respondents also reported altered sleeping habits (MF, 57 %; PV, 53 %; ET, 47 %). In addition, many respondents felt that their MPN affected their family/social life (MF, 79 %; PV, 63 %; ET, 55 %), relationship with their caregiver (MF, 28 %; PV, 18 %; ET, 15 %), and sex life (MF, 63 %; PV, 49 %; ET, 42 %).
Many respondents reported that their MPN limited productivity, including reduced work hours, calling in sick to work, and/or terminating their job (Table 4). Even respondents with low calculated prognostic risk scores reported calling in sick to work at least once in the preceding 30 days (Fig. 1). However, a consistent trend with regard to productivity and calculated prognostic risk scores was not observed across all 3 MPN subgroups. Greater proportions of respondents with PV who had low prognostic risk scores reported canceling planned activities and calling in sick to work compared with those who had high prognostic risk scores. Respondents in the high symptom severity quartile of all 3 MPN subgroups called in sick to work more often than respondents in the low symptom severity quartile.
Treatment management and therapies
The most important treatment goal reported by respondents with MF (42 %) or PV (25 %) was slowing or delaying progression of their disease; prevention of vascular/thrombotic events was the most important treatment goal reported by respondents with ET (35 %) (Table 5). A subset of respondents reported symptom relief as their most important treatment goal (MF, 7 %; PV, 9 %; ET, 9 %). In all MPN groups, fatigue was the most common symptom that respondents reported as the one they most wanted to resolve (MF, 47 %; PV, 33 %; ET, 33 %).
The therapies that respondents most often reported receiving at any time were aspirin (59 %), ruxolitinib (48 %), and hydroxyurea (42 %) in the MF group; phlebotomy (90 %), aspirin (83 %), and hydroxyurea (58 %) in the PV group; and aspirin (87 %), hydroxyurea (69 %), and anagrelide (36 %) in the ET group.
Discussion
The MPN Landmark survey is the first large survey to evaluate the experience of patients with MPNs in a contemporary US population and is the first study to extensively evaluate effects of MPNs on productivity and employment. The survey findings suggest that patients with MPNs experience a broad symptom burden and reductions in QoL, functional status, activities of daily living, and work productivity. These findings support recent reports of symptom burden and QoL that included non-US patient populations [4, 5, 17].
Increased recognition of the full disease burden associated with MPNs will help identify patients with unmet needs who may benefit from a change in management and is an important step toward improving patient care. Findings of this survey suggest that prognostic risk score may not capture all aspects of MPN disease burden. Notably, respondents with low prognostic risk scores reported experiencing disease burdens that may be underreported and underappreciated, highlighting an unmet need among patients with low prognostic risk scores. Mean MPN-SAF TSS values were actually higher (ie, more severe) in respondents with PV or ET who had low prognostic risk scores compared with those who had high risk scores. This discordance between prognostic risk score and MPN-SAF TSS was not observed in respondents with MF and may be explained by the inclusion of constitutional symptoms in the risk category calculation for MF but not PV or ET (Additional file 1: Table S1).
Symptoms related to MPNs are informative for early diagnosis and assessing patient clinical needs, but it is not uncommon for patients to experience symptoms well in advance of a formal diagnosis. Nearly one half of respondents with MF and the majority of respondents with PV or ET in the MPN Landmark survey reported experiencing MPN-related symptoms ≥1 year before diagnosis. The MPN Landmark survey also provided new and important data regarding the negative effects of MPNs on activities of daily living and work productivity and indicated that respondents with the most severe symptoms (ie, the highest symptom severity quartile) more frequently reported negative effects on QoL, productivity, and activities of daily living compared with the lowest quartile. In contrast, prognostic risk score was not consistently correlated with these measures of QoL and functionality. Improvements in symptom recognition and treatment may help ameliorate these negative effects.
Patient care in the MPN setting may be improved with updated management strategies. This study highlights the importance of using surveys or questionnaires—such as the MPN-SAF, the Cancer Support Source™ distress screening tool [18], or similar systematic approaches—to accurately capture patient-reported disease burden on a regular basis. Furthermore, participation in registries, such as the Cancer Experience Registry [19], may help communicate general patient symptoms and unmet needs to the broader field. Some symptomatic patients, including those with low prognostic risk scores, may benefit from a change in treatment. It will be important for physicians and researchers to optimize prognostic tools for identifying such patients and to evaluate potential biomarkers that could be used for making a targeted treatment change. For example, serum cytokines may be informative biomarkers for patients with MPNs. Levels of several serum cytokines are altered in patients with MPNs and have been correlated with disease characteristics, including symptoms and survival [20–22]. Further work will be required to validate these findings and determine if and how serum cytokine levels or other potential biomarkers could be used in clinical practice.
Limitations of the study were primarily a result of the descriptive design, self-reported nature of the survey, variations in respondent demographics, and challenges related to the relatively low prevalence of MPNs. The study was designed to be analyzed descriptively, which precluded statistical comparisons of the data. Because all results were self-reported by patient respondents, including treatments and risk factors used in the calculation of prognostic risk scores, data concerning symptom severity, outcomes, and comorbidities were not confirmed with clinical measures or respondents’ treating physicians. In addition, the sampling procedures may have introduced self-selection biases that could have affected the demographics of the respondents who participated. For example, relatively few low-risk respondents with MF or PV completed the survey; it remains unclear if this accurately represents the MPN population in the United States or if more severely affected patients with MF and PV were more motivated to participate. Respondents were predominantly college educated, with a mean annual household income > $75,000, compared with the median US household income in 2013, which was $52,250 [23]. In addition, although some symptom- and QoL-related questions were adapted from the validated MPN-SAF [5] and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire − Core 30 [24] instruments, the MPN Landmark survey itself did not include use of validated QoL instruments. As a result, the MPN Landmark survey may underrepresent the symptom burden experienced by the general MPN patient population. Because patients with MPNs are somewhat rare, the sample needed to be recruited by nonprobability sampling methods, which restricted the use of probability statistics to generate sample estimates. Notwithstanding these limitations, this was the only feasible methodology for assessing these rare conditions in a nationally distributed general population sample.
Conclusions
In conclusion, the MPN Landmark survey is the first large survey of its kind. As may be expected, patient respondents indicated that their most important treatment goals were to slow/delay disease progression and to prevent vascular/thrombotic events. However, the data also suggest that the disease burden experienced by patients with MPNs in the United States has been underreported in the literature and negatively affects QoL, activities of daily living, and the ability to work and be productive, including in patients with low prognostic risk scores and low symptom burden. MPN treatment considerations should include reducing the symptom burden as well as improving QoL and productivity to enhance the overall health and lives of patients with MPNs.
Abbreviations
- COPD:
-
chronic obstructive pulmonary disease
- ET:
-
essential thrombocythemia
- MF:
-
myelofibrosis
- MPN:
-
myeloproliferative neoplasm
- MPN-SAF:
-
Myeloproliferative Neoplasm Symptom Assessment Form
- MPN-SAF TSS:
-
Myeloproliferative Neoplasm Symptom Assessment Form 10-item total symptom score
- PV:
-
polycythemia vera
- QoL:
-
quality of life
- VA:
-
Veterans Affairs
References
Kim HJ, Choi CW, Won JH. The puzzle of myeloproliferative neoplasms: novel disease-specific mutations and new proposals for diagnostic criteria. Blood Res. 2014;49(4):211–3.
Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: when, which agent, and how? Blood. 2014;124(24):3529–37.
Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595–600.
Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–103.
Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–8.
Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, Derolf AR, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995–3001.
Falanga A, Marchetti M. Thrombosis in myeloproliferative neoplasms. Semin Thromb Hemost. 2014;40(3):348–58.
Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176–84.
Bjorkholm M, Hultcrantz M, Derolf AR. Leukemic transformation in myeloproliferative neoplasms: therapy-related or unrelated? Best Pract Res Clin Haematol. 2014;27(2):141–53.
Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly-annotated essential thrombocythemia, polycythemia vera and myelofibrosis. Blood. 2014;124(16):2507–13.
Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76.
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–8.
Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–81.
Passamonti F, Thiele J, Girodon F, Rumi E, Carobbio A, Gisslinger H, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood. 2012;120(6):1197–201.
Emanuel RM, Dueck AC, Geyer HL, Kiladjian J-J, Slot S, Zweegman S, et al. Myeloproliferative (MPN) symptom burden response thresholds: assessment of MPN-SAF TSS quartiles as potential markers of symptom response [abstract]. Blood (American Society of Hematology 55th Annual Meeting abstract). 2013;122(21):4067.
Geyer H, Scherber R, Kosiorek H, Dueck AC, Kiladjian JJ, Xiao Z, et al. Symptomatic Profiles of Patients With Polycythemia Vera: Implications of Inadequately Controlled Disease. J Clin Oncol. 2016;34:151–9.
Abelsson J, Andreasson B, Samuelsson J, Hultcrantz M, Ejerblad E, Johansson B, et al. Patients with polycythemia vera have the worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013;54(10):2226–30.
Miller MF, Mullins CD, Onukwugha E, Golant M, Buzaglo JS. Discriminatory power of a 25-item distress screening tool: a cross-sectional survey of 251 cancer survivors. Qual Life Res. 2014;23(10):2855–63.
Cancer Support Community. Cancer Experience Registry. 2015. https://www.cancerexperienceregistry.org/. Accessed 23 Feb 2016.
Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–63.
Vaidya R, Gangat N, Jimma T, Finke CM, Lasho TL, Pardanani A, et al. Plasma cytokines in polycythemia vera: phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. Am J Hematol. 2012;87(11):1003–5.
Pourcelot E, Trocme C, Mondet J, Bailly S, Toussaint B, Mossuz P. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: clinical implications. Exp Hematol. 2014;42(5):360–8.
Noss A. Household Income: 2013. American Community Survey Briefs. https://www.census.gov/content/dam/Census/library/publications/2014/acs/acsbr13-02.pdf. 2014. Accessed 23 Feb 2016.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Acknowledgments
Writing assistance was provided by Cory Pfeiffenberger, PhD (Complete Healthcare Communications, LLC, an ICON plc company), whose work was funded by Incyte Corporation. Assistance in the collection and analysis of MPN Landmark survey data was provided by Strategic Pharma Solutions Inc., whose work was funded by Incyte Corporation. The study was funded by Incyte Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
RM served as a consultant for Novartis and received research funding from Incyte Corporation, CTI BioPharma, Gilead, Genentech, Promedior, NS Pharma, and Pfizer. CBM served on speakers’ bureaus and received honoraria and research funding from Incyte Corporation. MT served on speakers bureaus for Incyte Corporation. JM served on an advisory committee for Incyte Corporation. SF served on speakers’ bureaus, served as a consultant, and received research funding and honoraria from Incyte Corporation and Gilead. XM served as a consultant for Incyte Corporation. WW received honoraria from Incyte Corporation. DCP and DGD are employees and stockholders of Incyte Corporation. JB is an employee and stockholder of ICF International. JOM received research funding paid to his institution from Incyte Corporation, Roche, Promedior, CTI BioPharma, Kalobios, and Novartis. SG declares that she has no competing interests.
Authors’ contributions
All authors read and approved the final manuscript for submission. RM, CBM, MT, SG, SF, JB, and JOM participated in designing and coordinating the study and drafting the manuscript. JM participated in analyzing data and reviewing and revising the manuscript. XM participated in designing and coordinating the study and reviewing and revising the manuscript. WW participated in designing the research plan and reviewing and revising the manuscript. DCP participated in analyzing data from the study. DGD participated in developing the study, analyzing data from the study, and drafting the manuscript.
Additional file
Additional file 1: Table S1.
Prognostic Risk Scoring Systems. Figure S1. Proportion of Respondents Who Reported Individual Symptoms to be Very Severe. (PDF 399 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mesa, R., Miller, C.B., Thyne, M. et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer 16, 167 (2016). https://doi.org/10.1186/s12885-016-2208-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2208-2