Abstract
Purpose
AML1/ETO fusion gene is one of disease-causing genes of t(8;21)-positive acute myeloid leukemia (AML). Oroxylin A (OA) has showed anticancer effects on other cancer cells. Here, studies were conducted to determine the antileukemia effect of OA on t(8;21)-positive AML cells in vitro and in vivo.
Materials and methods
The effects of OA on cell viability of t(8;21)-positive Kasumi-1 and primary AML cells were analyzed by MTT assay. Cell differentiation was examined by NBT reduction assay, flow cytometry analysis for CD11b/CD14, and Giemsa stain. Protein expressions were determined by Western blots. Immunofluorescence assay was used to verify the effect of OA on HDAC-1 expression in vivo. Immunohistochemical staining was applied to evaluate leukemic infiltration of AML-bearing NOD/SCID mice.
Results
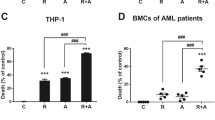
OA enhanced NBT reduction activity and CD11b/CD14 expression of AML1/ETO-positive AML cells markedly. Results of Giemsa staining also demonstrated that OA could induce the morphologic changes with reduction of nuclear/cytoplasmic ratios, suggesting the cell differentiation induced by OA. Further study showed that OA decreased the expression of fusion protein AML1/ETO and down-regulated HDAC-1 protein levels in vitro and in vivo. Moreover, OA increased the expression of differentiation-related proteins C/EBPα and P21. Acetylation levels of histones were also advanced obviously after treatment of OA. In vivo study indicated that OA could prolong the survival of AML-bearing NOD/SCID mice and reduce leukocytic infiltration of the spleen.
Conclusions
All these results suggested that OA might be a novel candidate agent for differentiation therapy for AML1/ETO-positive AML and the mechanism required further investigation.






Similar content being viewed by others
References
Abdel-Wahab O, Levine RL (2010) Recent advances in the treatment of acute myeloid leukemia. F1000 Med Rep 2:55
Atadja PW (2011) HDAC inhibitors and cancer therapy. Progress in drug research. Fortschritte der Arzneimittelforschung. Progres des recherches pharmaceutiques 67:175–195
Bassett SA, Barnett MP (2014) The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients 6:4273–4301
Bots M, Verbrugge I, Martin BP, Salmon JM, Ghisi M, Baker A, Stanley K, Shortt J, Ossenkoppele GJ, Zuber J, Rappaport AR, Atadja P, Lowe SW, Johnstone RW (2014) Differentiation therapy for the treatment of t(8;21) acute myeloid leukemia using histone deacetylase inhibitors. Blood 123:1341–1352
de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370:737–749
Farooqi AA, Tang JY, Li RN, Ismail M, Chang YT, Shu CW, Yuan SS, Liu JR, Mansoor Q, Huang CJ, Chang HW (2015) Epigenetic mechanisms in cancer: push and pull between kneaded erasers and fate writers. Int J Nanomed 10:3183–3191
Fredly H, Gjertsen BT, Bruserud O (2013) Histone deacetylase inhibition in the treatment of acute myeloid leukemia: the effects of valproic acid on leukemic cells, and the clinical and experimental evidence for combining valproic acid with other antileukemic agents. Clin Epigenet 5:12
Gao Y, Lu N, Ling Y, Chen Y, Wang L, Zhao Q, Qi Q, Liu W, Zhang H, You Q, Guo Q (2010) Oroxylin A inhibits angiogenesis through blocking vascular endothelial growth factor-induced KDR/Flk-1 phosphorylation. J Cancer Res Clin Oncol 136:667–675
Grant PA (2001) A tale of histone modifications. Genome Biol 2:0003
Guha M (2015) HDAC inhibitors still need a home run, despite recent approval. Nat Rev Drug Discov 14:225–226
Guibal FC, Alberich-Jorda M, Hirai H, Ebralidze A, Levantini E, Di Ruscio A, Zhang P, Santana-Lemos BA, Neuberg D, Wagers AJ, Rego EM, Tenen DG (2009) Identification of a myeloid committed progenitor as the cancer-initiating cell in acute promyelocytic leukemia. Blood 114:5415–5425
Hackanson B, Bennett KL, Brena RM, Jiang J, Claus R, Chen SS, Blagitko-Dorfs N, Maharry K, Whitman SP, Schmittgen TD, Lubbert M, Marcucci G, Bloomfield CD, Plass C (2008) Epigenetic modification of CCAAT/enhancer binding protein alpha expression in acute myeloid leukemia. Cancer Res 68:3142–3151
Hatlen MA, Wang L, Nimer SD (2012) AML1-ETO driven acute leukemia: insights into pathogenesis and potential therapeutic approaches. Front Med 6:248–262
Hui H, Chen Y, Yang H, Zhao K, Wang Q, Zhao L, Wang X, Li Z, Lu N, Guo Q (2014a) Oroxylin A has therapeutic potential in acute myelogenous leukemia by dual effects targeting PPARgamma and RXRalpha. Int J Cancer 134:1195–1206
Hui H, Yang H, Dai Q, Wang Q, Yao J, Zhao K, Guo Q, Lu N (2014b) Oroxylin A inhibits ATRA-induced IL-6 expression involved in retinoic acid syndrome by down-regulating CHOP. Gene 551:230–235
Kramer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, Brill B, Groner B, Bach I, Heinzel T, Gottlicher M (2003) The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J 22:3411–3420
Kramer OH, Muller S, Buchwald M, Reichardt S, Heinzel T (2008) Mechanism for ubiquitylation of the leukemia fusion proteins AML1-ETO and PML-RARalpha. FASEB J 22:1369–1379
Kretsovali A, Hadjimichael C, Charmpilas N (2012) Histone deacetylase inhibitors in cell pluripotency, differentiation, and reprogramming. Stem Cells Int 2012:184154
Lagace DC, Nachtigal MW (2004) Inhibition of histone deacetylase activity by valproic acid blocks adipogenesis. J Biol Chem 279:18851–18860
Loscalzo J, Handy DE (2014) Epigenetic modifications: basic mechanisms and role in cardiovascular disease (2013 Grover Conference series). Pulm Circ 4:169–174
Maeda T, Towatari M, Kosugi H, Saito H (2000) Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood 96:3847–3856
Mehdipour P, Santoro F, Minucci S (2015) Epigenetic alterations in acute myeloid leukemias. FEBS J 282:1786–1800
Newbold A, Matthews GM, Bots M, Cluse LA, Clarke CJ, Banks KM, Cullinane C, Bolden JE, Christiansen AJ, Dickins RA, Miccolo C, Chiocca S, Kral AM, Ozerova ND, Miller TA, Methot JL, Richon VM, Secrist JP, Minucci S, Johnstone RW (2013) Molecular and biologic analysis of histone deacetylase inhibitors with diverse specificities. Mol Cancer Ther 12:2709–2721
Nishii K, Usui E, Katayama N, Lorenzo FT, Nakase K, Kobayashi T, Miwa H, Mizutani M, Tanaka I, Nasu K, Dohy H, Kyo T, Taniwaki M, Ueda T, Kita K, Shiku H (2003) Characteristics of t(8;21) acute myeloid leukemia (AML) with additional chromosomal abnormality: concomitant trisomy 4 may constitute a distinctive subtype of t(8;21) AML. Leukemia 17:731–737
Peterson LF, Zhang DE (2004) The 8;21 translocation in leukemogenesis. Oncogene 23:4255–4262
Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276:36734–36741
Tabe Y, Jin L, Contractor R, Gold D, Ruvolo P, Radke S, Xu Y, Tsutusmi-Ishii Y, Miyake K, Miyake N, Kondo S, Ohsaka A, Nagaoka I, Andreeff M, Konopleva M (2007) Novel role of HDAC inhibitors in AML1/ETO AML cells: activation of apoptosis and phagocytosis through induction of annexin A1. Cell Death Differ 14:1443–1456
Tsuchiya Y, Ubara Y, Suwabe T, Hoshino J, Sumida K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Marui Y, Sawa N, Takemoto F, Takaichi K (2011) Successful treatment of acute promyelocytic leukemia in a patient on hemodialysis. Clin Exp Nephrol 15:434–437
Vangala RK, Heiss-Neumann MS, Rangatia JS, Singh SM, Schoch C, Tenen DG, Hiddemann W, Behre G (2003) The myeloid master regulator transcription factor PU.1 is inactivated by AML1-ETO in t(8;21) myeloid leukemia. Blood 101:270–277
Ververis K, Hiong A, Karagiannis TC, Licciardi PV (2013) Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biol Targets Ther 7:47–60
Volpe G, Walton DS, Del Pozzo W, Garcia P, Dasse E, O’Neill LP, Griffiths M, Frampton J, Dumon S (2013) C/EBPalpha and MYB regulate FLT3 expression in AML. Leukemia 27:1487–1496
Wakita S, Yamaguchi H, Miyake K, Mitamura Y, Kosaka F, Dan K, Inokuchi K (2011) Importance of c-kit mutation detection method sensitivity in prognostic analyses of t(8;21)(q22;q22) acute myeloid leukemia. Leukemia 25:1423–1432
Westendorf JJ, Yamamoto CM, Lenny N, Downing JR, Selsted ME, Hiebert SW (1998) The t(8;21) fusion product, AML-1-ETO, associates with C/EBP-alpha, inhibits C/EBP-alpha-dependent transcription, and blocks granulocytic differentiation. Mol Cell Biol 18:322–333
Witt O, Deubzer HE, Milde T, Oehme I (2009) HDAC family: what are the cancer relevant targets? Cancer Lett 277:8–21
Xu WS, Parmigiani RB, Marks PA (2007) Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26:5541–5552
Zhou Q, Melkoumian ZK, Lucktong A, Moniwa M, Davie JR, Strobl JS (2000) Rapid induction of histone hyperacetylation and cellular differentiation in human breast tumor cell lines following degradation of histone deacetylase-1. J Biol Chem 275:35256–35263
Zhou GB, Kang H, Wang L, Gao L, Liu P, Xie J, Zhang FX, Weng XQ, Shen ZX, Chen J, Gu LJ, Yan M, Zhang DE, Chen SJ, Wang ZY, Chen Z (2007) Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood 109:3441–3450
Zhuang S (2013) Regulation of STAT signaling by acetylation. Cell Signal 25:1924–1931
Zhuang WY, Cen JN, Zhao Y, Chen ZX (2013) Epigenetic silencing of Bcl-2, CEBPA and p14(ARF) by the AML1-ETO oncoprotein contributing to growth arrest and differentiation block in the U937 cell line. Oncol Rep 30:185–192
Acknowledgments
This work was supported by the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. G140042), Science Fund for Distinguished Young Scholars of Jiangsu province (BK20130024), the National Science and Technology Major Project (No. 2012ZX09304-001, 2012ZX09103101-050), the National Natural Science Foundation of China (Nos. 81300379, 81373449, and 81503096), Natural Science Foundation of Jiangsu province (No. BK20140668), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT-IRT1193), and Huahai Graduate Innovation Fund (CX13B-006HH), and the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Fundamental Research Funds for the Central Universities (PY2014YX0001; ZL2014YX0034).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Hui Hui and Xiaoxiao Zhang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hui, H., Zhang, X., Li, H. et al. Oroxylin A, a natural anticancer flavonoid compound, induces differentiation of t(8;21)-positive Kasumi-1 and primary acute myeloid leukemia cells. J Cancer Res Clin Oncol 142, 1449–1459 (2016). https://doi.org/10.1007/s00432-016-2160-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2160-1